DASATINIB
ダサチニブ水和物
BMS 354825
863127-77-9 HYDRATE, USAN, BAN INN, JAN
UNII: RBZ1571X5H
302962-49-8 FREE FORM Dasatinib anhydrous USAN, INN
Molecular Formula, C22-H26-Cl-N7-O2-S.H2-O, Molecular Weight, 506.0282
A pyrimidine and thiazole derived ANTINEOPLASTIC AGENT and PROTEIN KINASE INHIBITOR of BCR-ABL KINASE. It is used in the treatment of patients with CHRONIC MYELOID LEUKEMIA who are resistant or intolerant to IMATINIB.
An orally bioavailable synthetic small molecule-inhibitor of SRC-family protein-tyrosine kinases. Dasatinib binds to and inhibits the growth-promoting activities of these kinases. Apparently because of its less stringent binding affinity for the BCR-ABL kinase, dasatinib has been shown to overcome the resistance to imatinib of chronic myeloid leukemia (CML) cells harboring BCR-ABL kinase domain point mutations. SRC-family protein-tyrosine kinases interact with a variety of cell-surface receptors and participate in intracellular signal transduction pathways; tumorigenic forms can occur through altered regulation or expression of the endogenous protein and by way of virally-encoded kinase genes. (NCI Thesaurus)
5-Thiazolecarboxamide, N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)-1-piperazinyl)-2-methyl-4-pyrimidinyl)amino)-, monohydrate
Synthesis ReferenceUS6596746
DASATINIB ANHYDROUS
- KIN 001-5
- NSC 759877
- Sprycel
- 302962-49-8 Dasatinib anhydrous
- 5-THIAZOLECARBOXAMIDE, N-(2-CHLORO-6-METHYLPHENYL)-2-((6-(4-(2-HYDROXYETHYL)-1-PIPERAZINYL)-2-METHYL-4-PYRIMIDINYL)AMINO)-
- BMS-354825
- DASATINIB [INN]
- DASATINIB [MI]
- DASATINIB [WHO-DD]
- DASATINIB ANHYDROUS
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021986s000_Sprycel__ChemR.pdf
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021986_s001_s002.pdf
SPRYCEL (dasatinib) is an inhibitor of multiple tyrosine kinases.
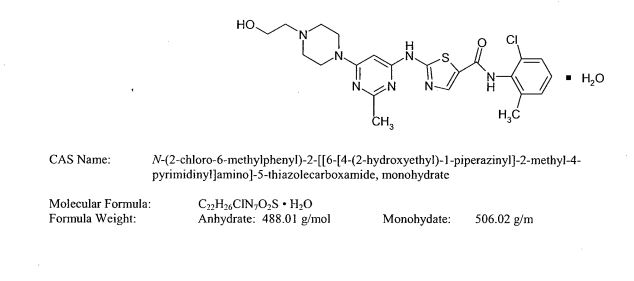
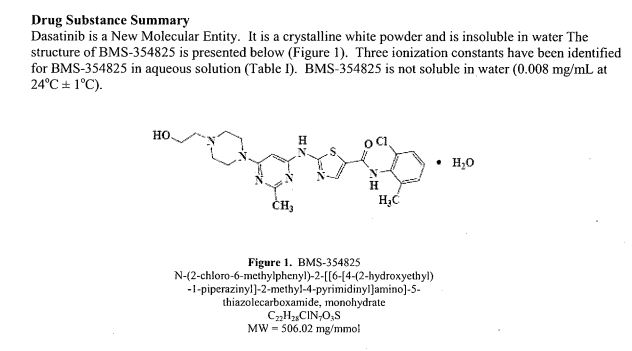
The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2- methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate. The molecular formula is C22H26ClN7O2S • H2O, which corresponds to a formula weight of 506.02 (monohydrate).
The anhydrous free base has a molecular weight of 488.01. Dasatinib has the following chemical structure: Dasatinib is a white to off-white powder and has a melting point of 280°–286° C.
The drug substance is insoluble in water and slightly soluble in ethanol and methanol. SPRYCEL tablets are white to off-white, biconvex, film-coated tablets containing dasatinib, with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol
|
|
- Clip
- https://www.pharmainbrief.com/files/2017/09/A-106-17-20170918-Reasons.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2016/202103Orig1s000ltr.pdf
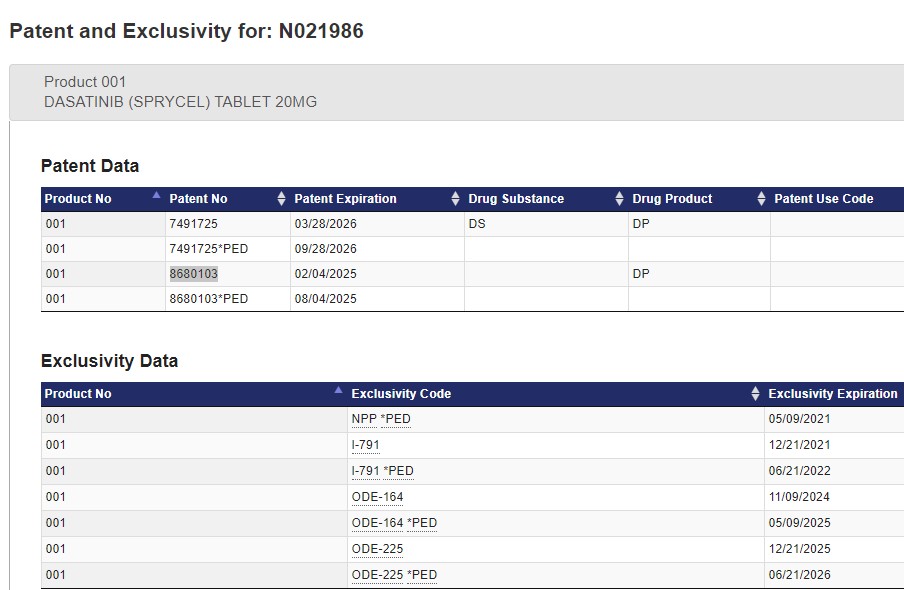
- U.S. Patent Number Expiration Date 6,596,746 (the ‘746 patent) June 28, 2020
- 7,125,875 (the ‘875 patent) April 13, 2020
- 7,153,856 (the ‘856 patent) April 28, 2020
- 7,491,725 (the ‘725 patent) March 28, 2026
- 8,680,103 (the ‘103 patent) February 4, 2025
- Drug Name:
- Dasatinib Hydrate
- Research Code:
- BMS-354825
- Trade Name:
- Sprycel®
- MOA:
- Kinase inhibitor
- Indication:
- Acute lymphoblastic leukaemia (ALL); Chronic myeloid leukemia (CML )
- Status:
- Approved
- Company:
- Bristol-Myers Squibb (Originator)
- Sales:
- $1,620 Million (Y2015);
$1,493 Million (Y2014);
$1,280 Million (Y2013);
$1,019 Million (Y2012);
$803 Million (Y2011); - ATC Code:
- L01XE06
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2006-06-28 | Marketing approval | Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet, Film coated | Eq. 20 mg/50 mg/70 mg/80 mg/100 mg/140 mg Dasatinib | Bristol-Myers Squibb | Priority; Orphan |
| 2006-06-28 | Additional approval | Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet, Film coated | 70 mg | Bristol-Myers Squibb | Priority |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2006-11-20 | Marketing approval | Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet, Film coated | 20 mg/50 mg/70 mg/80 mg/100 mg/140 mg | Bristol-Myers Squibb | Orphan |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2011-06-16 | Modified indication | Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet, Film coated | 20 mg/50 mg | Bristol-Myers Squibb, Otsuka | |
| 2009-01-21 | Marketing approval | Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet, Film coated | 20 mg/50 mg | Bristol-Myers Squibb, Otsuka |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2013-09-17 | Marketing approval | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 20 mg | 南京正大天晴制药 | ||
| 2013-09-17 | Marketing approval | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 50 mg | 南京正大天晴制药 | ||
| 2013-09-17 | Marketing approval | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 70 mg | 南京正大天晴制药 | ||
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 50 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 50 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 50 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 20 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 20 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 20 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 70 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 70 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 70 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 100 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 100 mg | Bristol-Myers Squibb | |
| 2011-09-07 | Marketing approval | 施达赛/Sprycel | Acute lymphoblastic leukaemia (ALL), Chronic myeloid leukemia (CML ) | Tablet | 100 mg | Bristol-Myers Squibb |
SPRYCEL (dasatinib) is a kinase inhibitor. The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate. The molecular formula is C22H26ClN7O2S • H2O, which corresponds to a formula weight of 506.02 (monohydrate). The anhydrous free base has a molecular weight of 488.01. Dasatinib has the following chemical structure:
 |
Dasatinib is a white to off-white powder. The drug substance is insoluble in water and slightly soluble in ethanol and methanol.
SPRYCEL tablets are white to off-white, biconvex, film-coated tablets containing dasatinib, with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.
Dasatinib hydrate was first approved by the U.S. Food and Drug Administration (FDA) on June 28, 2006, then approved by European Medicine Agency (EMA) on Nov 20, 2006, and approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on Jan 21, 2009. It was developed and marketed as Sprycel® by Bristol Myers Squibb in the US.
Dasatinibhydrate is a kinase inhibitor.It is indicated for the treatment ofchronic myeloid leukemia and acutelymphoblastic leukemia.
Sprycel® is available as film-coatedtabletfor oral use, containing 20, 50, 70, 80, 100 or 140 mg offreeDasatinib. The recommended dose is 100 mg once daily forchronic myeloid leukemia. Another dose is 140 mg once daily for accelerated phase chronic myeloid leukemia, myeloid or lymphoid blast phase chronic myeloid leukemia, or Ph+ acutelymphoblastic leukemia.
Dasatinib, also known as BMS-354825, is an orally bioavailable synthetic small molecule-inhibitor of SRC-family protein-tyrosine kinases. Dasatinib binds to and inhibits the growth-promoting activities of these kinases. Apparently because of its less stringent binding affinity for the BCR-ABL kinase, dasatinib has been shown to overcome the resistance to imatinib of chronic myeloid leukemia (CML) cells harboring BCR-ABL kinase domain point mutations.
Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL).[3] Specifically it is used to treat cases that are Philadelphia chromosome-positive (Ph+).[3] It is taken by mouth.[3]
Common adverse effects include low white blood cells, low blood platelets, anemia, swelling, rash, and diarrhea.[3] Severe adverse effects may include bleeding, pulmonary edema, heart failure, and prolonged QT syndrome.[3] Use during pregnancy may result in harm to the baby.[3] It is a tyrosine-kinase inhibitor and works by blocking a number of tyrosine kinases such as Bcr-Abl and the Src kinase family.[3]
Dasatinib was approved for medical use in the United States and in the European Union in 2006.[3][2] It is on the World Health Organization’s List of Essential Medicines.
Medical uses
Dasatinib is used to treat people with chronic myeloid leukemia and people with acute lymphoblastic leukemia who are positive for the Philadelphia chromosome.[5]
In the EU dasatinib is indicated for children with
- newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukaemia in chronic phase (Ph+ CML CP) or Ph+ CML CP resistant or intolerant to prior therapy including imatinib.[2]
- newly diagnosed Ph+ acute lymphoblastic leukaemia (ALL) in combination with chemotherapy.[2]
- newly diagnosed Ph+ CML in chronic phase (Ph+ CML-CP) or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib.[2]
and adults with
- newly diagnosed Philadelphia-chromosome-positive (Ph+) chronic myelogenous leukaemia (CML) in the chronic phase;[2]
- chronic, accelerated or blast phase CML with resistance or intolerance to prior therapy including imatinib mesilate;[2]
- Ph+ acute lymphoblastic leukaemia (ALL) and lymphoid blast CML with resistance or intolerance to prior therapy.[2]
Adverse effects
The most common side effects are infection, suppression of the bone marrow (decreasing numbers of leukocytes, erythrocytes, and thrombocytes),[6] headache, hemorrhage (bleeding), pleural effusion (fluid around the lungs), dyspnea (difficulty breathing), diarrhea, vomiting, nausea (feeling sick), abdominal pain (belly ache), skin rash, musculoskeletal pain, tiredness, swelling in the legs and arms and in the face, fever.[2] Neutropenia and myelosuppression were common toxic effects. Fifteen people (of 84, i.e. 18%) in the above-mentioned study developed pleural effusions, which was a suspected side effect of dasatinib. Some of these people required thoracentesis or pleurodesis to treat the effusions. Other adverse events included mild to moderate diarrhea, peripheral edema, and headache. A small number of people developed abnormal liver function tests which returned to normal without dose adjustments. Mild hypocalcemia was also noted, but did not appear to cause any significant problems. Several cases of pulmonary arterial hypertension (PAH) were found in people treated with dasatinib,[7] possibly due to pulmonary endothelial cell damage.[8]
On October 11, 2011, the U.S. Food and Drug Administration (FDA) announced that dasatinib may increase the risk of a rare but serious condition in which there is abnormally high blood pressure in the arteries of the lungs (pulmonary hypertension, PAH).[9] Symptoms of PAH may include shortness of breath, fatigue, and swelling of the body (such as the ankles and legs).[9] In reported cases, people developed PAH after starting dasatinib, including after more than one year of treatment.[9] Information about the risk was added to the Warnings and Precautions section of the Sprycel drug label.[9]
Pharmacology
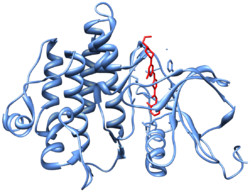
Crystal structure[10] (PDB 2GQG) of Abl kinase domain (blue) in complex with dasatinib (red).
Dasatinib is an ATP-competitive protein tyrosine kinase inhibitor. The main targets of dasatinib are BCR/Abl (the “Philadelphia chromosome”), Src, c-Kit, ephrin receptors, and several other tyrosine kinases.[11] Strong inhibition of the activated BCR-ABL kinase distinguishes dasatinib from other CML treatments, such as imatinib and nilotinib.[11][12] Although dasatinib only has a plasma half-life of three to five hours, the strong binding to BCR-ABL1 results in a longer duration of action.[12]
History
Dasatinib was developed by collaboration of Bristol-Myers Squibb and Otsuka Pharmaceutical Co., Ltd,[13][14][15] and named for Bristol-Myers Squibb research fellow Jagabandhu Das, whose program leader says that the drug would not have come into existence had he not challenged some of the medicinal chemists‘ underlying assumptions at a time when progress in the development of the molecule had stalled.[16]
Society and culture
Legal status
Dasatinib was approved for used in the United States in June 2006 and in the European Union in November 2006[17][2]
In October 2010, dasatinib was approved in the United States for the treatment of newly diagnosed adults with Philadelphia chromosome positive chronic myeloid leukemia in chronic phase (CP-CML).[18]
In November 2017, dasatinib was approved in the United States for the treatment of children with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in the chronic phase.[19]
Approval was based on data from 97 pediatric participants with chronic phase CML evaluated in two trials—a Phase I, open-label, non-randomized, dose-ranging trial and a Phase II, open-label, non-randomized trial.[19] Fifty-one participants exclusively from the Phase II trial were newly diagnosed with chronic phase CML and 46 participants (17 from the Phase I trial and 29 from the Phase II trial) were resistant or intolerant to previous treatment with imatinib.[19] The majority of participants were treated with dasatinib tablets 60 mg/m2 body surface area once daily.[19] Participants were treated until disease progression or unacceptable toxicity.[19]
Economics
The Union for Affordable Cancer Treatment objected to the price of dasatinib, in a letter to the U.S. trade representative. The average wholesale price in the U.S. is $367 per day, twice the price in other high income countries. The price in India, where the average annual per capita income is $1,570, and where most people pay out of pocket, is Rs6627 ($108) a day. Indian manufacturers offered to supply generic versions for $4 a day, but, under pressure from the U.S., the Indian Department of Industrial Policy and Promotion refused to issue a compulsory license.[20]
Bristol-Myers Squibb justified the high prices of cancer drugs with the high R&D costs, but the Union of Affordable Cancer Treatment said that most of the R&D costs came from the U.S. government, including National Institutes of Health funded research and clinical trials, and a 50% tax credit. In England and Wales, the National Institute for Health and Care Excellence recommended against dasatinib because of the high cost-benefit ratio.[20]
The Union for Affordable Cancer Treatment said that “the dasatinib dispute illustrates the shortcomings of US trade policy and its impact on cancer patients”[20]
Brand names
In Bangladesh dasatinib is available under the trade name Dasanix by Beacon Pharmaceuticals.In India, It is marketed by brand name NEXTKI by EMCURE PHARMACEUTICALS[medical citation needed]
Research
Dasatinib has been shown to eliminate senescent cells in cultured adipocyte progenitor cells.[21] Dasatinib has been shown to induce apoptosis in senescent cells by inhibiting Src kinase, whereas quercetin inhibits the anti-apoptotic protein Bcl-xL.[21] Administration of dasatinib along with quercetin to mice improved cardiovascular function and eliminated senescent cells.[22] Aged mice given dasatinib with quercetin showed improved health and survival.[22]
Giving dasatinib and quercetin to mice eliminated senescent cells and caused a long-term resolution of frailty.[23] A study of fourteen human patients suffering from idiopathic pulmonary fibrosis (a disease characterized by increased numbers of senescent cells) given dasatinib and quercetin showed improved physical function and evidence of reduced senescent cells.[21]
1. WO2005077945A2 / US2012302750A1.
1. WO0062778A1 / US6596746B1.
1. CN104292223A.
1. CN103420999A.
Syn 1
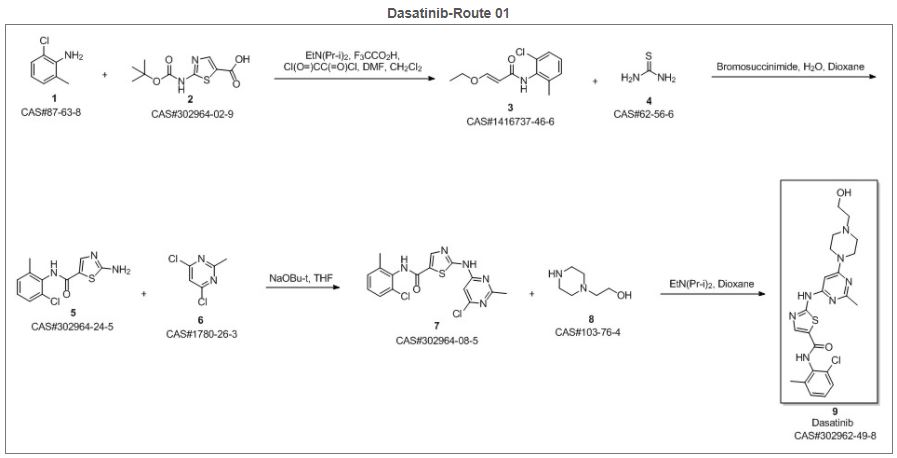
Reference
Balaji, N.; Sultana, Sayeeda. Trace level determination and quantification of potential genotoxic impurities in dasatinib drug substance by UHPLC/infinity LC. International Journal of Pharmacy and Pharmaceutical Sciences. Department of Chemistry. St. Peter’s University. Tamil Nadu, India 600054. Volume 8. Issue 10. Pages 209-216. 2016
SYN 2
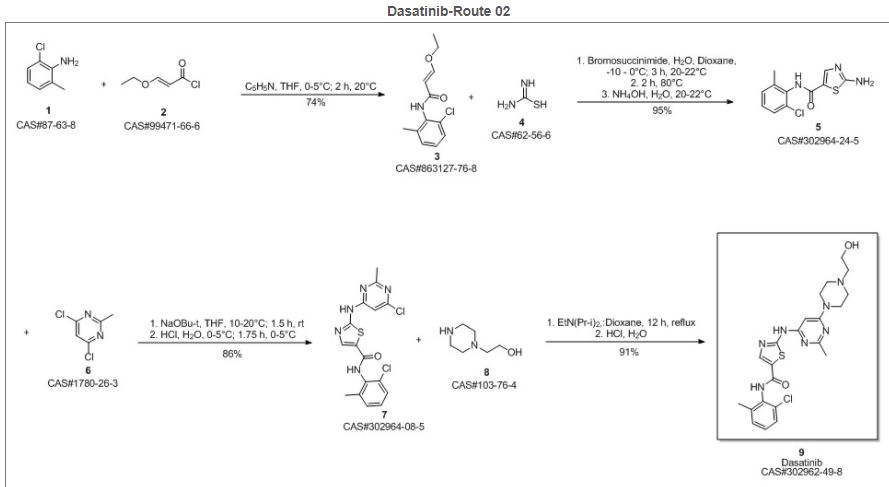
Reference
Zhang, Shaoning; Wei, Hongtao; Ji, Min. Synthesis of dasatinib. Zhongguo Yiyao Gongye Zazhi. Dept. of Pharmaceutical Engineering, School of Chemistry & Chemical Engineering. Southeast University. Nanjing, Jiangsu Province, Peop. Rep. China 210096. Volume 41. Issue 3. Pages 161-163. 2010
SYN 3
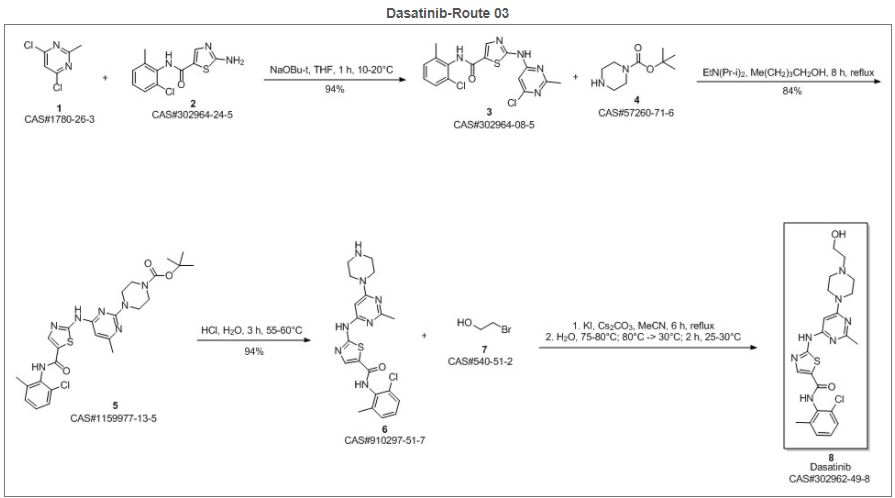
Reference
Suresh, Garbapu; Nadh, Ratnakaram Venkata; Srinivasu, Navuluri; Yennity, Durgaprasad. A convenient new and efficient commercial synthetic route for dasatinib (Sprycel). Synthetic Communications. Division of Chemistry, Department of Science and Humanities. Vignan’s Foundation for Science Technology and Research University. Guntur, India. Volume 47. Issue 17. Pages 1610-1621. 2017
SYN 4
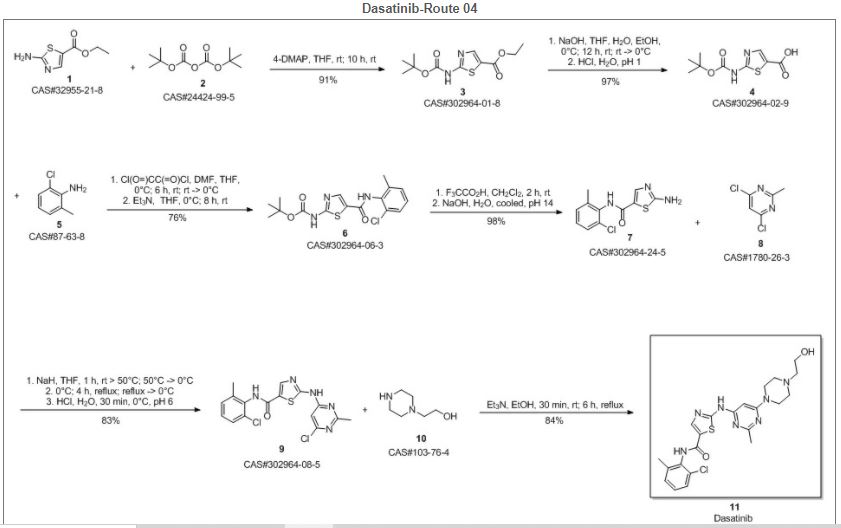
Reference
Chen, Bang-Chi; Zhao, Rulin; Wang, Bei; Droghini, Roberto; Lajeunesse, Jean; Sirard, Pierre; Endo, Masaki; Balasubramanian, Balu; Barrish, Joel C. A new and efficient preparation of 2-aminothiazole-5-carbamides: applications to the synthesis of the anticancer drug dasatinib. ARKIVOC (Gainesville, FL, United States). Discovery Chemistry. Bristol-Myers Squibb Research and Development. Princeton, USA 08543. Issue 6.Pages 32-38. 2010
SYN 5
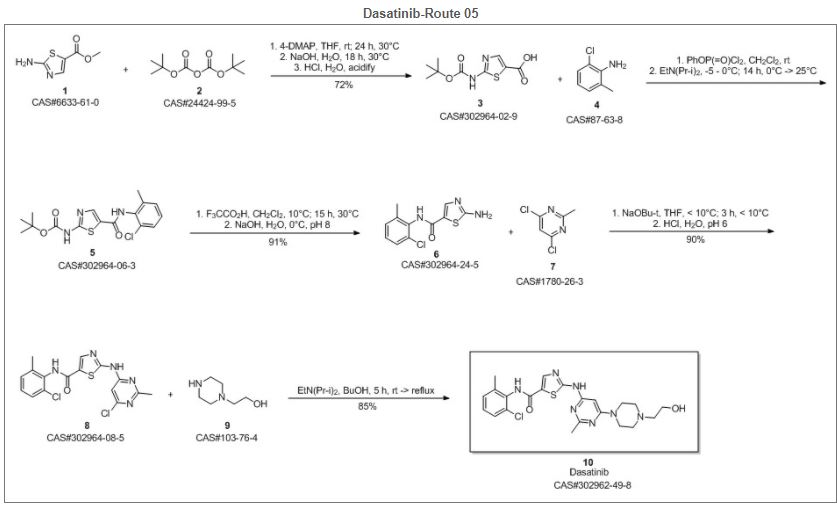
Reference
An, Kang; Guan, Jianning; Yang, Hao; Hou, Wen; Wan, Rong. Improvement on the synthesis of Dasatinib. Jingxi Huagong Zhongjianti. College of Science. Nanjing University of Technology. Nanjing, Jiangsu Province, Peop. Rep. China 211816. Volume 41. Issue 2. Pages 42-44. 2011
PATENT
https://patents.google.com/patent/US7491725B2/en
EXAMPLESExample 1
(S)-1-sec-Butylthiourea
Example 2
(R)-1-sec-Butylthiourea
Example 3
3B. Example 3
Example 4
Example 5
N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (The Compound of Formula (IV))
5A. 1-(6-Chloro-2-methylpyrimidin-4-yl)thiourea
5B. (E)-N-(2-Chloro-6-methylphenyl)-3-ethoxyacrylamide
5C. 2-Amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
5D. 2-(6-Chloro-2-methylpyrimidin-4-ylamino)-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
5E. Example 5
Example 6
N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
Example 7
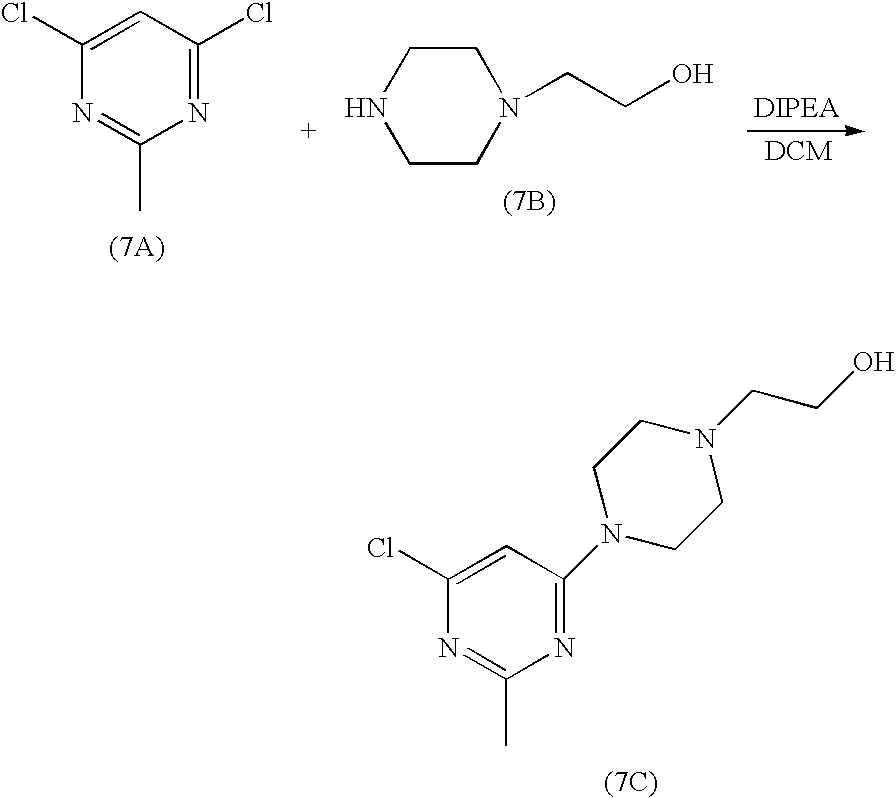
N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide7A. 2-[4-(6-Chloro-2-methyl-pyrimidin-4-yl)-piperazin-1-yl]-ethanol
7B. Example 7
To a 250 ml of round bottom flask were charged compound 5C (1.9 g, 7.1 mmol), compound 7C (1.5 g, 5.9 mmol), K2CO3 (16 g, 115.7 mmol), Pd (OAc)2 (52 mg, 0.23 mmol) and BINAP (291 mg, 0.46 mmol). The flask was placed under vacuum and flushed with nitrogen. Toluene was added (60 ml). The suspension was heated to 100-110° C. and stirred at this temperature for 20h. After cooling to room temperature, the mixture was applied to a silica gel column. The column was first eluted with EtOAC, and then with 10% of MeOH in EtOAC. Finally, the column was washed with 10% 2M ammonia solution in MeOH/90% EtOAC. The fractions which contained the desired product were collected and concentrated to give compound IV as a yellow solid (2.3 g).
Analytical Methods
Example 8
crystalline monohydrate of N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV)
- Charge 48 g of the compound of formula (IV).
- Charge approximately 1056 mL (22 mL/g) of ethyl alcohol, or other suitable alcohol.
- Charge approximately 144 mL of water.
- Dissolve the suspension by heating to approximately 75° C.
- Optional: Polish filter by transfer the compound of formula (IV) solution at 75° C. through the preheated filter and into the receiver.
- Rinse the dissolution reactor and transfer lines with a mixture of 43 mL of ethanol and 5 mL of water.
- Cool to 70° C. and maintain 70° C. for ca. 1 h.
- Cool from 70 to 5 C over 2 h, and maintain the temperature between 0 at 5° C. for at least 2 h.
- Filter the crystal slurry.
- Wash the filter cake with a mixture of 96 mL of ethanol and 96 mL of water.
- Dry the material at ≦50° C. under reduced pressure until the water content is 3.4 to 4.1% by KF to afford 41 g (85 M %).
Alternately, the monohydrate can be obtained by:- 1) An aqueous solution of the acetate salt of compound IV was seeded with monohydrate and heated at 80° C. to give bulk monohydrate.
- 2) An aqueous solution of the acetate salt of compound IV was seeded with monohydrate. On standing several days at room temperature, bulk monohydrate had formed.
- 3) An aqueous suspension of compound IV was seeded with monohydrate and heated at 70° C. for 4 hours to give bulk monohydrate. In the absence of seeding, an aqueous slurry of compound IV was unchanged after 82 days at room temperature.
- 4) A solution of compound IV in a solvent such as NMP or DMA was treated with water until the solution became cloudy and was held at 75-85° C. for several hours. Monohydrate was isolated after cooling and filtering.
- 5) A solution of compound IV in ethanol, butanol, and water was heated. Seeds of monohydrate were added to the hot solution and then cooled. Monohydrate was isolated upon cooling and filtration.
-
- a(Å)=13.862(1);
- b(Å)=9.286(1);
- c(Å)=38.143(2);
Example 9
crystalline n-butanol solvate of N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV)
Example 10
crystalline ethanol solvate of N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV)
Example 11
crystalline N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV) (Neat form N-6)
Example 12
crystalline N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV) (neatform T1H1-7)
wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase. They are expected to be useful in the treatment of protein tyrosine kinase-associated disorders such as immunologic and oncological disorders [see, U.S. Pat. No. 6,596,746 (the ‘746 patent), assigned to the present assignee and incorporated herein by reference], and p38 kinase-associated conditions such as inflammatory and immune conditions, as described in U.S. patent application Ser. No. 10/773,790, filed Feb. 6, 2004, claiming priority to U.S. Provisional application Ser. No. 60/445,410, filed Feb. 6, 2003 (hereinafter the ‘410 application), both of which are also assigned to the present assignee and incorporated herein by reference.
then, hydrolyzing the resulting bromothiazole esters to the corresponding carboxylic acids and converting the acids to the corresponding acyl chlorides, e.g.,
then finally, coupling the acyl chlorides with anilines to afford bromothiazole-benzamide intermediates which were further elaborated to aminothiazole-benzamide final products, e.g.,
SUMMARY OF THE INVENTION
wherein L, Ar, R2, R3, R4, R5, and m are as defined below, comprising reacting a compound having the formula (II),
wherein Q is the group —O—P*, wherein P* is selected so that, when considered together with the oxygen atom to which P* is attached, Q is a leaving group, and Ar, L, R2, R3, and m are as defined below,
with a halogenating reagent in the presence of water followed by a thiourea compound having the formula (III),
EXAMPLESExample 1
(S)-1-sec-Butylthiourea
Example 2
(R)-1-sec-Butylthiourea
Example 3
3B. Example 3
Example 4
Example 5
N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (The compound of Formula (IV))
5A. 1-(6-Chloro-2-methylpyrimidin-4-yl)thiourea
5B. (E)-N-(2-Chloro-6-methylphenyl)-3-ethoxyacrylamide
5C. 2-Amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
5D. 2-(6-Chloro-2-methylpyrimidin-4-ylamino)-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
5E. Example 5
Example 6
N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
Example 7
N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide7A. 2-[4-(6-Chloro-2-methyl-pyrimidin-4-yl)-piperazin-1-yl]-ethanol
7B. Example 7
Analytical Methods
Example 8
Crystalline monohydrate of N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV)
-
- b(Å)=9.286(1);
- c(Å)=38.143(2);
Example 9
Crystalline n-butanol solvate of N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV)
Example 10
Crystalline ethanol solvate of N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV)
Example 11
Crystalline N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV) (Neat form N-6)
Example 12
Crystalline N-(2-chloro-6-methylphenyl)-2-(6-(4-(3-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (IV) (neat form T1H1-7)
2-Aminothiazole (1) was discovered as a novel Src family kinase inhibitor template through screening of our internal compound collection. Optimization through successive structure−activity relationship iterations identified analogs 2 (Dasatinib, BMS-354825) and 12m as pan-Src inhibitors with nanomolar to subnanomolar potencies in biochemical and cellular assays. Molecular modeling was used to construct a putative binding model for Lck inhibition by this class of compounds. The framework of key hydrogen-bond interactions proposed by this model was in agreement with the subsequent, published crystal structure of 2 bound to structurally similar Abl kinase. The oral efficacy of this class of inhibitors was demonstrated with 12m in inhibiting the proinflammatory cytokine IL-2 ex vivo in mice (ED50 ∼ 5 mg/kg) and in reducing TNF levels in an acute murine model of inflammation (90% inhibition in LPS-induced TNFα production when dosed orally at 60 mg/kg, 2 h prior to LPS administration). The oral efficacy of 12m was further demonstrated in a chronic model of adjuvant arthritis in rats with established disease when administered orally at 0.3 and 3 mg/kg twice daily. Dasatinib (2) is currently in clinical trials for the treatment of chronic myelogenous leukemia.

PATENT
https://patents.google.com/patent/WO2019209908A1/en
Scheme 1 shows a general process for the preparation of Dasatinib as disclosed in US 2006/0004067. Intermediate 3 and N-(2-hydroxyethyl) piperazine are heated together in a solvent system comprising n-butanol as a solvent and diisopropyl ethylamine (DIPEA) as a base. On cooling of the reaction mixture, Dasatinib precipitates out which is isolated by filtration.
References
- ^ “Sprycel (Dasatinib)” (PDF). Therapeutic Goods Administration(TGA). Retrieved 18 July 2020.
- ^ Jump up to:a b c d e f g h i j “Sprycel EPAR”. European Medicines Agency(EMA). Retrieved 28 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b c d e f g h “Dasatinib”. The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Keating GM (January 2017). “Dasatinib: A Review in Chronic Myeloid Leukaemia and Ph+ Acute Lymphoblastic Leukaemia”. Drugs. 77 (1): 85–96. doi:10.1007/s40265-016-0677-x. PMID 28032244. S2CID 207489056.
- ^ Olivieri, A.; Manzione, L. (2007). “Dasatinib: a new step in molecular target therapy”. Annals of Oncology. 18 Suppl 6: vi42–vi46. doi:10.1093/annonc/mdm223. PMID 17591830.
- ^ “NHS – Healthcare News”. nelm.nhs.uk. Archived from the original on 5 May 2013. Retrieved 27 September 2011.
- ^ Yurttaş NO, Eşkazan AE (2018). “Dasatinib-induced pulmonary arterial hypertension”. British Journal of Clinical Pharmacology. 84 (5): 835–845. doi:10.1111/bcp.13508. PMC 5903230. PMID 29334406.
- ^ Jump up to:a b c d “Sprycel (dasatinib) and risk of pulmonary arterial hypertension”. U.S. Food and Drug Administration (FDA). 23 September 2011. Retrieved 28 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Tokarski JS, Newitt JA, Chang CY, Cheng JD, Wittekind M, Kiefer SE, et al. (June 2006). “The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants”. Cancer Research. 66 (11): 5790–7. doi:10.1158/0008-5472.CAN-05-4187. PMID 16740718.
- ^ Jump up to:a b Piscitani L, Sirolli V, Morroni M, Bonomini M (2020). “Nephrotoxicity Associated with Novel Anticancer Agents (Aflibercept, Dasatinib, Nivolumab): Case Series and Nephrological Considerations”. International Journal of Molecular Sciences. 21(14): e4878. doi:10.3390/ijms21144878. PMC 7402330. PMID 32664269.
- ^ Jump up to:a b Braun TP, Eide CA, Druker BJ (2020). “Response and Resistance to BCR-ABL1-Targeted Therapies”. Cancer Cell. 37(4): 530–542. doi:10.1016/j.ccell.2020.03.006. PMC 7722523. PMID 32289275.
- ^ “Otsuka and Bristol-Myers Squibb Announce a Change in Contract Regarding Collaboration in Japan in the Oncology Therapy Area”.
- ^ “FDA Approves U.S. Product Labeling Update for Sprycel (dasatinib) to Include Three-Year First-Line and Five-Year Second-Line Efficacy and Safety Data in Chronic Myeloid Leukemia in Chronic Phase”. Bristol-Myers Squibb (Press release).
- ^ “Bristol-Myers Squibb Announces Extension of U.S. Agreement for ABILIFY and Establishment of an Oncology Collaboration with Otsuka”. Bristol-Myers Squibb (Press release).
- ^ Drahl C (16 January 2012). “How Jagabandhu Das made dasatinib possible”. The Safety Zone blog. Chemical & Engineering News. Retrieved 29 August 2016.
- ^ “Drug Approval Package: Sprycel (Dasatinib) NDA #021986 & 022072”. U.S. Food and Drug Administration (FDA). 6 September 2006. Retrieved 28 April 2020.
- ^ “2010 Notifications”. U.S. Food and Drug Administration (FDA). 18 November 2010. Retrieved 28 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b c d e “FDA approves dasatinib for pediatric patients with CML”. U.S. Food and Drug Administration (FDA). 9 November 2017. Retrieved 28 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b c Cohen D (November 2014). “US trade rep is pressing Indian government to forbid production of generic cancer drug, consortium says”. BMJ. 349: g6593. doi:10.1136/bmj.g6593. PMID 25370846. S2CID 206903723.
- ^ Jump up to:a b c Kirkland JL, Tchkonia T (2020). “Senolytic drugs: from discovery to translation”. Journal of Internal Medicine. 288 (5): 518–536. doi:10.1111/joim.13141. PMC 7405395. PMID 32686219.
- ^ Jump up to:a b Paez-Ribes M, González-Gualda E, Doherty GJ, Muñoz-Espín D (2019). “Targeting senescent cells in translational medicine”. EMBO Molecular Medicine. 11 (12): e10234. doi:10.15252/emmm.201810234. PMC 6895604. PMID 31746100.
- ^ Wyld L, Bellantuono I, Tchkonia T, Danson S, Kirkland JL (2020). “Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies”. Cancers. 12 (8): e2134. doi:10.3390/cancers12082134. PMC 7464619. PMID 32752135.
Further reading[edit]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. (December 2004). “Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays”. Journal of Medicinal Chemistry. 47 (27): 6658–61. doi:10.1021/jm049486a. PMID 15615512.
External links
- “Dasatinib”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Sprycel, Dasanix |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607063 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 96% |
| Metabolism | Liver |
| Elimination half-life | 1.3 to 5 hours |
| Excretion | Fecal (85%), kidney (4%) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.228.321 |
| Chemical and physical data | |
| Formula | C22H26ClN7O2S |
| Molar mass | 488.01 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
/////////////DASATINIB, BMS 35482503, KIN 001-5, NSC 759877, Sprycel, BMS, APOTEX, ダサチニブ水和物 , X78UG0A0RN, дазатиниб , دازاتينيب , 达沙替尼 ,
#DASATINIB, #BMS 35482503, #KIN 001-5, #NSC 759877, #Sprycel, #BMS, #APOTEX, #ダサチニブ水和物 , #X78UG0A0RN, #дазатиниб , #دازاتينيب , #达沙替尼 ,
O.Cc1nc(Nc2ncc(s2)
PATENT
https://patents.google.com/patent/US8884013B2/en
The following items of products prepared by Method A were detected: microscope-crystal form (See. FIG. 1); XRPD Test (See. FIG. 2), IR Test (See. FIG. 3), DSC-TGA Test (See. FIG. 4-1, 4–2), 13C Solid-state NMR Test (See. FIG. 5).
INDUSTRIAL APPLICATION