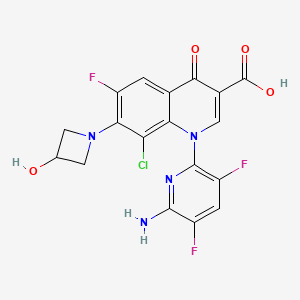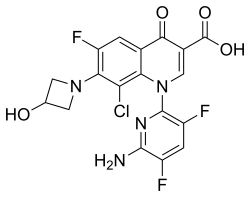
Delafloxacin
- Molecular FormulaC18H12ClF3N4O4
- Average mass440.760 Da
Delafloxacin, ABT-492, RX-3341, WQ-3034, A-319492
Delafloxacin is a Fluoroquinolone Antibacterial. The chemical classification of delafloxacin is Fluoroquinolones.


Delafloxacin (INN) (trade name Baxdela) is a fluoroquinolone antibiotic used to treat acute bacterial skin and skin structure infections.[1] It was developed and marketed by Melinta Therapeutics (formerly Rib-X Pharmaceuticals),[1] which subsequently merged with Cempra.[2]

syn
CN 104876911

Medical use
Delafloxacin is used to treat acute bacterial skin and skin structure infections caused by designated susceptible bacteria.[1]
Susceptible bacteria are:[1]
- Gram-positive organisms: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus anginosus group, Streptococcus pyogenes, and Enterococcus faecalis
- Gram-negative organisms: Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
It has not been tested in pregnant women.[1]
Adverse effects
Like other drugs in the fluoroquinolone class, delafloxacin contains a black box warning about the risk of tendinitis, tendon rupture, peripheral neuropathy, central nervous system effects, and exacerbation of myasthenia gravis. The label also warns against the risk of hypersensitivity reactions and Clostridium difficile-associated diarrhea.[1]
Adverse effects occurring in more than 2% of clinical trial subjects included nausea, diarrhea, headache, elevated transaminases, and vomiting.[1]

Interactions
Like other fluoroquinolones, delafloxacin chelates metals including aluminum, magnesium, sucralfate, iron, zinc, and divalent and trivalent cations like didanosine; using this drugs with antacids, some dietary supplements, or drugs buffered with any of these ions will interfere with available amounds of delafloxacin.[1]
Pharmacology
The half-life varies in around 8 hours at normal doses. Excretion is 65% through urine, mostly in unmetabolized form, and 28% via feces. Clearance is reduced in people with severe kidney disease.[3]
Delafloxacin is more active (lower MIC90) than other quinolones against Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). In contrast to most approved fluoroquinolones, which are zwitterionic, delafloxacin has an anionic character, which results in a 10-fold increase in delafloxacin accumulation in both bacteria and cells at acidic pH. This property is believed to confer to delafloxacin an advantage for the eradication of Staphylococcus aureus in acidic environments, including intracellular infections.[3]
Chemistry
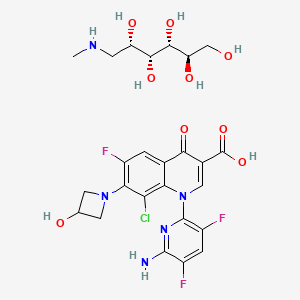
The chemical name is 1-Deoxy-1 (methylamino)-D-glucitol, 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl) 4-oxo-1,4-dihydroquinoline-3-carboxylate (salt).[1]
The injectable form of delafloxacin is sold as the meglumine salt of the active ingredient and its United States Adopted Name, delafloxacin meglumine, reflects that; the injection formulation also includes EDTA and sulfobutylether-β-cyclodextrin. The tablet is made of delafloxacin, citric acid anhydrous, crospovidone, magnesium stearate, microcrystalline cellulose, povidone, sodium bicarbonate, and sodium phosphate monobasic monohydrate.[1]
History
Delafloxacin was known as ABT-492, RX-3341, and WQ-3034 while it was under development.[4]
Rib-X Pharmaceuticals acquired delafloxacin from Wakunaga Pharmaceutical in 2006.[5] Rib-X was renamed to Melinta Therapeutics in 2013.[6]
Key clinical trials for delafloxacin have been performed by Melinta regarding indications for skin and skin structure infections as well as complicated bacterial infections and uncomplicated gonorrhea. The trial on gonorrhea was terminated before data was released.[7]
Delafloxacin was approved by the FDA in June 2017, after it was noninferior to vancomycin plus aztreonam in two trials on 1042 patients with acute bacterial skin and skin structure infection.[8] New Drug Applications (NDA) for delafloxacin (Baxdela) 450 mg tablets and 300 mg injections were approved by the FDA in June 2017.[9]
The FDA obligated Melinta to conduct further studies as follows:[9]
- a 5-year surveillance study to determine if resistance emerges, with the final report due in December 2022
- a study of the IV form in pregnant rats to determine distribution to the reproductive tract, due June 2018, with further studies required if there is significant distribution.
Melinta merged with Cempra in August, 2017.[2]
Melinta has entered into commercialization and distribution agreements with both Menarini Therapeutics (March 2017) and Eurofarma Laboratórios (January 2015) for international commercialization of delafloxacin. The agreement with Menarini allows them to commercialize and distribute in 68 countries, including Europe, China, and South Korea among others. A similar agreement with Eurofarma allows for commercialization in Brazil.[7]
PATENT
de Iaf Ioxacin Preparation
[0101] was added to the S-neck flask resultant product of Example 11 (3.5 Yap, dirty 〇1 0.76) implemented 01. (35 blood) milky white suspension, was added glacial acetic acid (3. OmL), stirred at room temperature to embrace completely clear solution was added dropwise distilled water 70 fed blood, filter, wash coating, evaporated to dryness to give a pale yellow powder 3. Og, purity 99.8% (HPLC), m / z (MH + M41.03, IH NMR (400MHz, DMSO) S4.20 (m, 2H), 4.45 (m, lH), 4.61 (m, 2H), 5.63 (d, lH), 6.69 (s, 2H), 7.81 (d, lH), 7.95 (dd, lH), 8.69 (d, lH), 14.34 (brs, lH).
PAPER
Org. Process Res. Dev. 2006, 10, 803-807.
Chlorination at the 8-Position of a Functionalized Quinolone and the Synthesis of Quinolone Antibiotic ABT-492
The total synthesis of quinolone antibiotic ABT-492 has been achieved in 67% yield over nine steps from 2,4,5-trifluorobenzoic acid. The highlights of this synthesis include a novel chemoselective chlorination at the 8-position of a highly elaborated quinolone core. In addition, a Lewis acid promoted cyclization reaction to form the quinolone heterocycle was developed which was incorporated into a one-pot, three-step cyclization/coupling/protection sequence that proceeds in 93% yield.
1-(6-Amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (ABT-492), NCS Process: . Mp: 238−241 °C. 1H NMR (CDCl3) δ 14.63 (brs, 1H), 8.70 (d, J = 0.7 Hz, 1H), 7.95 (dd, J = 9.9, 0.7 Hz, 1H), 7.83 (d, J = 13.6 Hz, 1H), 6.75 (s, 2H), 5.75 (d, J = 5.8 Hz, 1H), 4.61 (m, 12H), 4.47 (m, 1H), 4.18 (m, 2H). Anal. Calcd for C18H12ClF3N4O4: C, 49.05; H, 2.74; N, 12.71. Found: C, 48.90; H, 2.48; N, 12.62.
PATENT
EXAMPLE 5
A solution of 2,4,5-trifluorobenzoic acid (139.5Kg) in DMF (8.4Kg) and toluene (613Kg) was treated with thionyl chloride (139.4Kg), stirred at 60°C for 3.5 hours, cooled to 250C, concentrated to 20% of its original volume, treated with toluene (600Kg), distilled and stored at ambient temperature.
EXAMPLE 6
A suspension of potassium ethyl malonate (50.8Kg) and magnesium chloride
(34.5Kg) in toluene (130Kg) below 00C was treated with THF (265L), cooled to 0°C, treated with triethylamine (75Kg), warmed to 5O0C, stirred for 1-5 hours, cooled to 00C, treated with 22% (w/w) of EXAMPLE 5 in toluene (163Kg), warmed to ambient temperature, stirred for 2 hours, added to 2M HCl (407Kg), stirred for 30 minutes, separated from the water layer and washed with water. This procedure was repeated, and the organic layers were combined, concentrated with an ethanol (150L) azeotrope, treated with water (30% by weight of the organic layer), stirred for 3 hours at 00C, and filtered. The andfiltrant was washed with 3:1 ethanol/water and dried under vacuum at 35-45°C to provide 86Kg of product. H NMR (CDCl3) (keto) δ 7.75 (ddd, J=10.8, 10.8, 6.0Hz, IH), 7.02 (ddd, IH), 4.27 (q, J=7.2Hz, 2H), 3.95 (d, 4.2Hz, 2H), 1.35 (t, J=7.3Hz, 3H); (enol) δ 12.72 (s, IH), 7.85 (ddd, J=10.5, 9.6, 6.6Hz, IH), 6.96 (ddd, J=10.5, 10.5, 6.6Hz, IH), 5.84 (s, IH), 4.23 (q, J=7.2Hz, 2H), 1.27 (t, J=7.4Hz, 3H).
EXAMPLE 7A
A solution of EXAMPLE 6 (83.2Kg) in triethyl orthoformate (80.1Kg) at reflux was stirred for 0.5-1 hour, treated with acetic anhydride (103.5Kg), stirred for 12 hours and cooled to ambient temperature to provide a solution that was used immediately.
EXAMPLE 7B
The solution of EXAMPLE 7A was treated with N-methylpyrrolidinone (210Kg), acetonitrile (161Kg) and water (3Kg), added to a suspension of EXAMPLE 4 (57.4Kg) in 1 : 1 N-methylpyrrolidinone (210Kg) and acetonitrile (161Kg), stirred for 2 hours, added to water (662Kg) and filtered. The fϊltrant was washed with (2:1) acetonitrile/water and water and dried under vacuum at 600C to provide 119.5Kg of product. Mp 157-16O0C; 1H NMR (CDCl3, 300 MHz) (E) δ 1.15 (t, 3H), 4.16 (q, 2H), 4.64 (br s, 2H), 6.90 (m, IH), 7.22 (t, IH), 7.32 (m, IH), 9.03 (d, IH), 12.44 (bd, IH); (Z) δ 1.03 (t, 3H), 4.11 (q, 2H), 4.60 (br s, 2H), 6.90 (m, IH), 7.20 (t, IH), 7.48 (m, IH), 8.90 (d, IH), 11.17 (bd, IH).
EXAMPLE 8A
A mixture of EXAMPLE 7 (115Kg) and lithium chloride (24.3Kg) in
N-methylpyrrolidinone (769Kg) below 350C was treated with DBU (946.1Kg) and stirred for 2 hours to provide a solution of EXAMPLE 8 A that was used immediately.
EXAMPLE 8B
The solution of EXAMPLE 8A below 4O0C was treated with EXAMPLE 2 (33.9Kg) and DBU (109Kg) and stirred for 2-5 hours to provide a solution of EXAMPLE 8B that was used immediately.
EXAMPLE 8C
The solution of EXAMPLE 8B was treated with isobutyric anhydride (99.7Kg), stirred at 350C for 1-2 hours, cooled to 20-300C, treated with ethyl acetate (104Kg) and 10% aqueous citric acid (570Kg) and filtered. The filtrant was washed with water and dried under vacuum at 500C to provide 136Kg of product. 1H NMR (DMSO-d6, 400 MHz) δ 8.49 (s, IH), 8.00 (dd, J=9.0, 9.3 Hz, IH), 7.75 (d, J=12.8 Hz, IH), 6.79 (br s, 2H), 5.95 (dd, J=I.5, 7.6 Hz, IH), 5.21 (m, IH), 4.36 (t, J=7.4 Hz, 2H), 4.02 (q, J=7.0 Hz, 2H), 3.95 (dd, J=3.7, 9.2 Hz, 2H), 2.58 (hept, J=7.0 Hz, IH), 1.26 (t, J=7.0 Hz, 3H), 1.11 (d, J=7.0 Hz, 6H).
EXAMPLE 10
A solution of N-chlorosuccinimide (25.3Kg) in methyl acetate (419Kg) at 170C was treated with sulfuric acid (560 g), transferred to a slurry of EXAMPLE 8 (92.7Kg) in ethyl acetate (244Kg) at 17°C while maintaining the reaction temperature at 17°C,
quenched/washed with 1.5% aqueous sodium bicarbonate (370Kg), washed with
10% aqueous sodium sulfite (200Kg) and concentrated. The concentrate was dissolved in isopropanol, treated with 4% (w/w) aqueous potassium hydroxide (750Kg), stirred at 5O0C until hydrolysis was complete, passed through a polishing filter, treated with 12% aqueous acetic acid (410Kg) and filtered. The filtrant was washed with water and dried at 5O0C to provide 73Kg of product. 1H NMR (CDCl3) δ 14.63 (brs, IH), 8.70 (d, J=0.7Hz, IH), 7.95 (dd, J=9.9, 0.7Hz, IH), 7.83 (d, J=13.6Hz, IH), 6.75 (s, 2H), 5.75 (d, J=5.8Hz, IH), 4.61 (m, 12H), 4.47 (m, IH), 4.18 (m, 2H).
PATENT
https://www.google.com/patents/CN104876911A?cl=en

Currently, 德拉沙 star for the synthesis mainly in the following two ways:
[0004] 1, Chinese patent CN1201459A _2,4,5_ trifluorobenzoyl from 3-chloro-ethyl ester synthesis De Lasha star. Used in this reaction is N, N- dimethylformamide high temperature and potassium carbonate cyclization, prone to impurities, after cyclization is hydrolyzed required, increase the reaction step, a low yield. Reaction scheme is as follows:
[0005]
[0006] 2, published in the Journal of Organic Chemistry (Org Process Res & Dev2006,4, 751) provides a new synthesis method 德拉沙 star from 2,4,5_ trifluoroacetic acid as the starting material, synthetic Germany Lassa star. This reaction because of the need in eight selective chlorination, so 7-hydroxy need protection, reaction step increase. And when eight were chlorinated 7 substituent easily broken, harsh reaction conditions, the reaction yield is low, is not suitable for mass production. Reaction scheme is as follows:
[0007]

Example: 8_-Chloro-6-fluoro-1- (6-amino-3,5-difluoro-2-yl) -7- (3-hydroxy-1-azetidinyl) – 1,4-dihydro-4-oxo-3-quinolinecarboxylic acid (Dela Sha star) Synthesis of
[0025] 3-chloro-2,4,5-trifluoro-benzoyl acetate (78,0.025111〇1) in 501,111 flask, triethylorthoformate (5. 9g, 0. 04mol) and vinegar anhydride, heated at reflux for 3h ~ 5h, evaporated under reduced pressure excess triethyl orthoformate and acetic anhydride, was added N- methylpyrrolidone was diluted, and then 2,6-diamino-3,5-difluoro-pyridine was suspended ( 3. 8g, 0. 026mol) and N- methylpyrrolidone were suspended, was added dropwise to the above solution, after completion of the reaction was added anhydrous lithium chloride (2. 6g) and DBU (4.6g, 0.03mol) (1 1,8-diazabicyclo [5.4.0] undec-ene _7_) was heated with stirring, HPLC monitored the reaction was complete. Then 3-hydroxy-azetidine hydrochloride (3. 52g) was added to the above solution was added dropwise DBU, the reaction was continued to completion. In the aqueous solution of isopropanol and potassium hydroxide, heating the hydrolysis, the hydrolysis is completed after adjusting PH = 3 solid precipitated. Filtering, washing, to give a yellow solid (7. 82g), yield 71%.
[0026] MP: 238-241 ° C
[0027] Tuen bandit 1 (square)?! (: 13) 14.32 0 ^ 8,1 1), 8.51 ((1, J = 0.7Hz, lH), 7.96 (dd, J = 9 · 9,0 · 7Ηζ , 1H), 7 · 64 (d, J = 13. 6Hz, 1H), 6 · 92 (s, 2H), 5 · 86 (d, J = 5. 8Hz, 1H), 4 · 89 (m, 12H ), 4 · 32 (m, 1H), 4 · 18 (m, 2H).
syn


SYN
Synthetic Description
Reference: Zhang, Guimin; Wang, Xiujuan; Zhao, Xuliang; Wu, Suzhen. Method for preparation of delafloxacin. CN 107778293. (Assignee Lunan Pharmaceutical Group Corp., Peop. Rep. China)

Delafloxacin – Synthetic route 1

Synthetic Description
Reference: Roger Hanselmann, Graham Johnson, Maxwell M. Reeve, and Shu-Ting Huang. Identification and Suppression of a Dimer Impurity in the Development of Delafloxacin. Organic Process Research & Development 2009 13 (1), 54-59. DOI: 10.1021/op800238q
Delafloxacin – Synthetic route 2

Delafloxacin – Synthetic route 3

Delafloxacin – Synthetic route 4

Delafloxacin – Synthetic route 6

Delafloxacin – Synthetic route 7

Synthetic Description
Reference: Pan, Yuan; Zuo, Xiaoyong; Zhang. Preparation method of highly purified delafloxacin meglumine. CN 106256824. (Assignee Chongqing Pharmaceutical Research Institute Co., Ltd., Peop. Rep. China)
References
- ^ Jump up to:a b c d e f g h i j “Delafloxacin tablets US label” (PDF). FDA. June 2017. Retrieved July 9,2017.
 This article incorporates text from this source, which is in the public domain. For label updates, see FDA index page for NDA 208610 for tablets, and see FDA index page for NDA 208611 for injectable form.
This article incorporates text from this source, which is in the public domain. For label updates, see FDA index page for NDA 208610 for tablets, and see FDA index page for NDA 208611 for injectable form. - ^ Jump up to:a b “Cempra Press Releases”.
- ^ Jump up to:a b Candel, FJ; Peñuelas, M (2017). “Delafloxacin: design, development and potential place in therapy”. Drug design, development and therapy. 11: 881–891. doi:10.2147/DDDT.S106071. PMC 5367733
 . PMID 28356714.
. PMID 28356714. - Jump up^ “Delafloxacin”. AdisInsight. Retrieved 10 July 2017.
- Jump up^ Cartwright, Heather (12 July 2011). “Rib-X Pharmaceuticals Signs Global Antibiotic Research Collaboration with Sanofi”. PharmaDeals Review (7). doi:10.3833/pdr.v2011i7.1494. Archived from the original on 25 April 2012.
- Jump up^ Stearns, John (August 1, 2016). “Melinta Therapeutics takes aim at deadly drug-resistant bacteria”. Hartford Business Journal.
- ^ Jump up to:a b Markham, Anthony (July 2017). “Delafloxacin: First Global Approval” Check
|url=value (help). Drugs. 77: 1481–1486 – via Springer. - Jump up^ Osborne, Randy (20 June 2017). “Melinta’s I.V., oral delafloxacin wins FDA nod in skin infections”. BioWorld.
- ^ Jump up to:a b “NDA Approval Letter: NDA 208610 and NDA 208611” (PDF). FDA. June 19, 2017.
-
Cited Patent Filing date Publication date Applicant Title CN1201459A * Sep 20, 1996 Dec 9, 1998 涌永制药株式会社 Novel pyridonecarboxylic acid derivatives or their salts and antibacterial agent comprising same as active ingredient JP2005097116A * Title not available WO2006015194A2 * Jul 29, 2005 Feb 9, 2006 Abbott Laboratories Preparation of pyridonecarboxylic acid antibacterials -
FDA Orange Book Patents
FDA Orange Book Patents: 1 of 8 (FDA Orange Book Patent ID) Patent 8871938 Expiration Sep 23, 2029 Applicant MELINTA Drug Application N208610 (Prescription Drug: BAXDELA. Ingredients: DELAFLOXACIN MEGLUMINE) FDA Orange Book Patents: 2 of 8 (FDA Orange Book Patent ID) Patent 9539250 Expiration Oct 7, 2025 Applicant MELINTA Drug Application N208611 (Prescription Drug: BAXDELA. Ingredients: DELAFLOXACIN MEGLUMINE) FDA Orange Book Patents: 3 of 8 (FDA Orange Book Patent ID) Patent 7728143 Expiration Nov 20, 2027 Applicant MELINTA Drug Application N208611 (Prescription Drug: BAXDELA. Ingredients: DELAFLOXACIN MEGLUMINE)
 |
|
| Clinical data | |
|---|---|
| Trade names | Baxdela |
| Synonyms | ABT-492; RX-3341; WQ-3034 |
| Routes of administration |
Oral, intravenous injection |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H12ClF3N4O4 |
| Molar mass | 440.76 g/mol |
| 3D model (JSmol) | |
/////////////Delafloxacin, ABT-492, RX-3341, WQ-3034, FDA 2017, A-319492
C1C(CN1C2=C(C=C3C(=C2Cl)N(C=C(C3=O)C(=O)O)C4=NC(=C(C=C4F)F)N)F)O