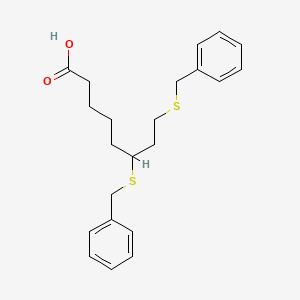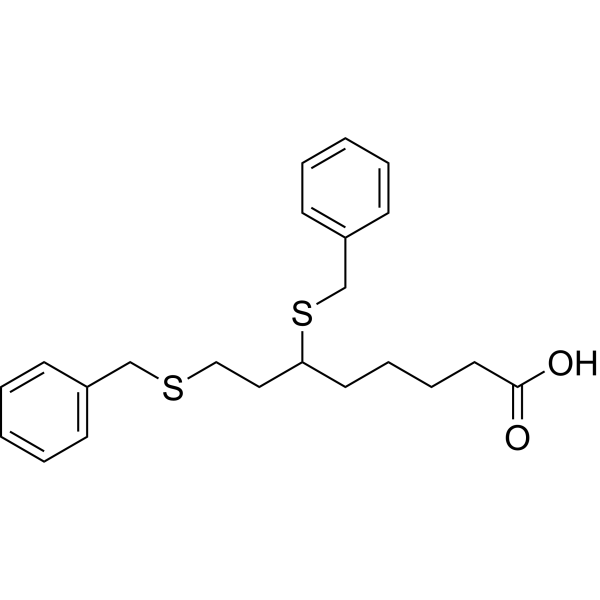

Devimistat
CPI-613
| Molecular Weight |
388.59 |
|---|---|
| Formula |
C₂₂H₂₈O₂S₂ |
| CAS No. |
95809-78-2 |
| SMILES |
O=C(O)CCCCC(SCC1=CC=CC=C1)CCSCC2=CC=CC=C2 |
phase III, hematological cancer
6,8-Bis(benzylsulfanyl)octanoic acid
Octanoic acid, 6,8-bis[(phenylMethyl)thio]-
Octanoic acid, 6,8-bis((phenylmethyl)thio)-
Rafael Pharmaceuticals (formerly Cornerstone Pharmaceuticals), a subsidiary of Rafael Holdings, is developing devimistat, the lead candidate from a program of thioctans and their derivatives that act as pyruvate dehydrogenase and alpha-ketoglutarate inhibitors and stimulators of pyruvate dehydrogenase kinase (PDK), using the company’s proprietary Altered Energy Metabolism Directed (AEMD) platform, for the iv treatment of hematological cancer [phase III, January 2021].
Devimistat (INN; development code CPI-613) is an experimental anti-mitochondrial drug being developed by Rafael Pharmaceuticals.[1] It is being studied for the treatment of patients with metastatic pancreatic cancer and relapsed or refractory acute myeloid leukemia (AML).
Devimistat’s mechanism of action differs from other drugs, operating on the tricarboxylic acid cycle and inhibiting enzymes involved with cancer cell energy metabolism. A lipoic acid derivative different from standard cytotoxic chemotherapy, devimistat is currently being studied in combination with modified FOLFIRINOX to treat various solid tumors and heme malignancies.
Regulation
The U.S. Food and Drug Administration (FDA) has designated devimistat as an orphan drug for the treatment of pancreatic cancer, AML, myelodysplastic syndromes (MDS), peripheral T-cell lymphoma, and Burkitt’s lymphoma, and given approval to initiate clinical trials in pancreatic cancer and AML.
Clinical trials
Clinical trials of the drug are underway including a Phase III open-label clinical trial[2] to evaluate efficacy and safety of devimistat plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas.
Developed as part of Rafael’s proprietary Altered Metabolism Directed (AMD) drug platform, CPI-613® was discovered at Stony Brook University. CPI-613® is designed to target the mitochondrial tricarboxylic acid (TCA) cycle, an indispensable process essential to tumor cell multiplication and survival, selectively in cancer cells.
The attacks of CPI-613® on the TCA cycle also substantially increases the sensitivity of cancer cells to a diverse range of chemotherapeutic agents. This synergy allows for combinations of CPI-613® with lower doses of these generally toxic drugs to be highly effective with lower patient side effects. Combinations with CPI-613® represent a diverse range of potential opportunities to substantially improve patient benefit in many different cancers.
The U.S. Food and Drug Administration (FDA) has given Rafael approval to initiate pivotal clinical trials in pancreatic cancer and acute myeloid leukemia (AML), and has designated CPI-613® as an orphan drug for the treatment of pancreatic cancer, AML, Myelodysplastic syndromes (MDS), peripheral T-cell lymphoma and Burkitt’s lymphoma. The EMA has granted orphan drug designation to CPI-613® for pancreatic cancer and AML.
Learn more about recent developments involving CPI-613®: CPI-613® (devimistat) Fact Sheet
he FDA granted a Fast Track designation to devimistat for the treatment of patients with acute myeloid leukemia.
The FDA has granted a Fast Track designation to devimistat (CPI-613) for the treatment of patients with acute myeloid leukemia (AML), Rafael Pharmaceuticals, announced in a press release.1
“This designation underscores the pressing need to find new ways to combat this aggressive disease,” said Jorge Cortes, MD, director of the Georgia Cancer Center at Augusta University, and principal investigator on the phase 3 clinical trial, in a statement. “It brings hope not only to clinicians, but to patients who hear that they have been diagnosed.”
The first-in-class agent devimistat targets enzymes that are involved in cancer cell energy metabolism. This therapy substantially increases the sensitivity of cancer cells to a diverse range of chemotherapies, and this synergy allows for potential combinations that could be more effective with devimistat and lower doses of drugs that are generally toxic.
“Receiving Fast Track designation, especially during a pandemic that has created significant challenges for many trials across the globe, is a testament to the dedicated work of the Rafael team,” stated Sanjeev Luther, president and CEO of Rafael Pharmaceuticals, Inc.
Devimistat combinations appear promising with a diverse range of potential opportunities to improve benefit in patients with various cancer types. Two pivotal phase 3 clinical trials, including the AVENGER 500 study in pancreatic cancer (NCT03504423) and ARMADA 2000 for AML (NCT03504410), have been approved for initiation by the FDA.
The primary end point of the multicenter, open-label, randomized ARMADA 2000 study is complete response (CR), and secondary end points include overall survival and CR plus CR with partial hematologic recovery rate. To be eligible to enroll to the study, patients must be aged ≥50 years with a documented AML diagnosis that has relapsed from or became refractory to previous standard therapy. Patients must have an ECOG performance status of 0 to 2 and an expected survival longer than 3 months.
Five hundred patients are expected to be enrolled and randomized in the study. To enroll, patients could not have received prior radiotherapy or cytotoxic chemotherapy for their current AML. Those with active central nervous system involvement, active uncontrolled bleeding, history of other malignancy, or known hypersensitivity to study drugs are ineligible to enroll to the trial as well.
This study aims to determine the safety and efficacy of devimistat in combination with high-dose cytarabine and mitoxantrone in older patients with relapsed/refractory AML compared with high-dose cytarabine and mitoxantrone therapy alone. Other control groups include patients treated with mitoxantrone, etoposide, and cytarabine and the combination of fludarabine, cytarabine, and filgrastim. The addition of devimistat is expected to improve the CR rate in patients who are aged 50 years or older with relapsed/refractory AML.
In a prior phase 1 study of devimistat plus high-dose cytarabine and mitoxantrone in patients with relapsed/refractory AML, the addition of devimistat sensitized AML cells to chemotherapy treatment.2
The objective response rate was 50% including CRs in 26 of 62 evaluable patients. Median overall survival was 6.7 months. In patients above age 60, the CR or CR with incomplete hematologic recovery rate was 47% and the median survival was 6.9 months.
This designation for this experimental anti-mitochondrial agent follows news of another Fast Track designation granted to devimistat for the treatment of patients with metastatic pancreatic cancer in November 2020, as well as an Orphan Drug designation granted in October 2020 for the treatment of patients with soft tissue sarcoma.
References
1. Rafael Pharmaceuticals Receives FDA Fast Track Designation for CPI-613® (devimistat) for the treatment of acute myeloid leukemia (AML). News Release. Rafael Pharmaceuticals, Inc. December 15, 2020. Accessed December 15, 2020. https://bit.ly/34g6YsR
2. Pardee TS, Anderson RG, Pladna KM, et al. A Phase I Study of CPI-613 in Combination with High-Dose Cytarabine and Mitoxantrone for Relapsed or Refractory Acute Myeloid Leukemia. Clin Cancer Res. 2018;24(9):2060-2073. doi:10.1158/1078-0432.CCR-17-2282
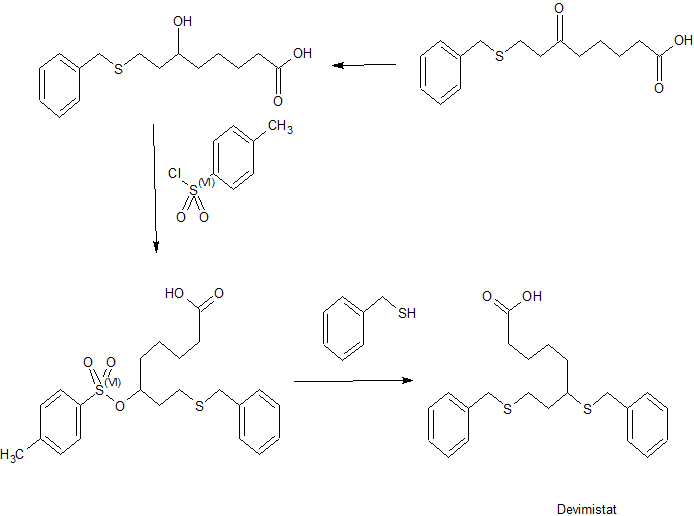
PATENT
WO-2021011334
Deuterated derivatives of 6,8-bis(benzylsulfanyl)octanoic acid (CPI-613 or devimistat ) or its salts for treating cancer.
CPI-613 (6,8-bis(benzylsulfanyl)octanoic acid) is a first-in-class investigational small-molecule (lipoate analog), which targets the altered energy metabolism unique to many cancer cells. CPI-613 is currently being evaluated in two phase III clinical trials, and has been granted orphan drug designation for the treatment of pancreatic cancer, acute myeloid leukemia (AML), peripheral T-cell lymphoma (PTCL), Burkitt lymphoma and myelodysplastic syndromes (MDS).
[0004] One limitation to the clinical utility of CPI-613 is its very rapid metabolism. After IV dosing the half-life of 6,8-bis(benzylsulfanyl)octanoic acid is only about 1-2 hours (Pardee,
T.S. et al, Clin Cancer Res. 2014, 20, 5255-64). The short half-life limits the patient’s overall exposure to the drug and necessitates administration of relatively high doses. For safety reasons, CPI-613 is administered via a central venous catheter as an IV infusion over 30-120 minutes, with higher doses requiring longer infusion times.
The terms“6,8-bis(benzylsulfanyl)octanoic acid” and“ 6,8-bis-benzylthio-octanoic acid” refer to the compound known as CPI-613 or devimistat, having the chemical structure
PATENT
WO2020132397
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020132397&_cid=P22-KKC2YP-45863-1
claiming the use of CPI-613 in combination with an autophagy inhibitor eg chloroquine for treating eg cancers.
CPI-613 (6,8-bis-benzylthio-octanoic acid) is a first-in-class investigational small-molecule (lipoate analog), which targets the altered energy metabolism that is common to many cancer cells. CPI-613 has been evaluated in multiple phase I, I/II, and II clinical studies, and has been granted orphan drug designation for the treatment of pancreatic cancer, acute myeloid leukemia (AML), peripheral T-cell lymphoma (PTCL), Burkitt lymphoma and myelodysplastic syndromes (MDS).
PAPER
https://pubs.acs.org/doi/10.1021/op200091t
An Efficient, Economical Synthesis of the Novel Anti-tumor Agent CPI-613
References
- ^ “CPI-613”. Rafael Pharmaceuticals.
- ^ Philip PA, Buyse ME, Alistar AT, Rocha Lima CM, Luther S, Pardee TS, Van Cutsem E (October 2019). “A Phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas”. Future Oncology. 15 (28): 3189–3196. doi:10.2217/fon-2019-0209. PMC 6854438. PMID 31512497.
 |
|
| Clinical data | |
|---|---|
| Other names | CPI-613 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.231.125 |
| Chemical and physical data | |
| Formula | C22H28O2S2 |
| Molar mass | 388.58 g·mol−1 |
| 3D model (JSmol) | |
//////////devimistat, CPI-613, CPI 613, phase 3, hematological cancer , Fast Track designation, ORPHAN DRUG,