Diethyl Chlorophosphate (CAS no 814–49–3)(15)
Preparative Procedure
In a dry four-neck 250 mL round-bottom flask equipped with a mechanical stirrer, a thermometer, and an argon inlet were introduced at 20 °C triethylamine (21.3 g, 210 mmol), tert-butyl methyl ether (75 mL), and ethanol (9.67 g, 210 mmol). Phosphorus oxychloride (15.3 g, 100 mmol) was added dropwise in 10 min via a syringe; the temperature was maintained below −5 °C. Then, the cooling bath was removed and the white suspension was vigorously stirred during 5 h at 20 °C. The mixture was filtered and the solid was rinsed with 3 × 100 mL of diethyl ether. The solvents were removed under vacuo. The resulting crudez product was distilled under reduced pressure to afford 15.4 g (89% yield) of diethyl chlorophosphate containing 3% of triethylphosphate.
Eb3 = 51 °C (lit:(3) Eb10 = 85–87 °C).
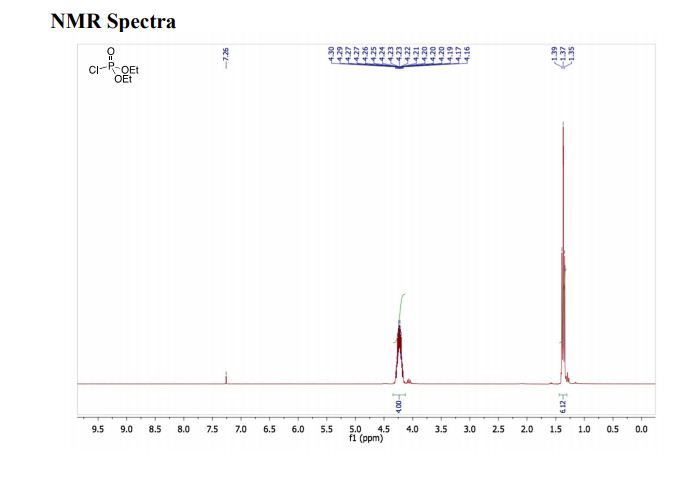
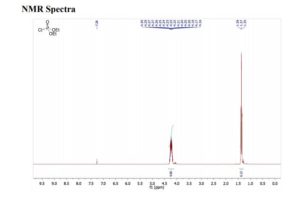
1H NMR (CDCl3, 300 MHz, δ): 1.37 (6H, t, J = 6.0 Hz), 4.16–4.30 (4H, m).

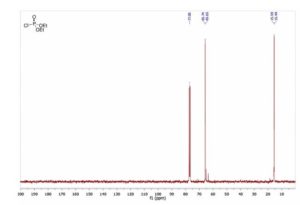
13C NMR (CDCl3, 75 MHz, δ): 15.6 (d, J = 7.5 Hz), 65.7 (d, J = 6.8 Hz).
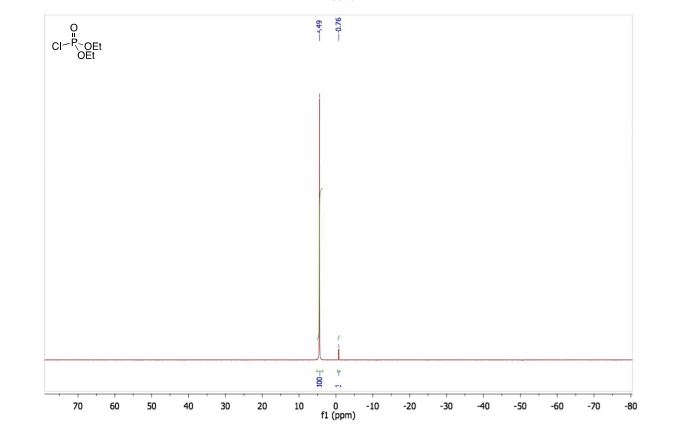
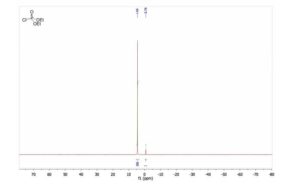
31P RMN (CDCl3, 121 MHz, δ): 4.5.
15Acharya, J.; Gupta, A. K.; Shakya, P. D.; Kaushik, M. P. Tetrahedron Lett. 2005, 46, 5293 DOI: 10.1016/j.tetlet.2005.06.024
Eco-Friendly and Industrially Scalable Synthesis of the Sex Pheromone of Lobesia botrana. Important Progress for the Eco-Protection of Vineyard
† Chimie ParisTech, PSL Research University, CNRS, Institut de Recherche de Chimie Paris (IRCP), F-75005 Paris, France
‡ M2i, Route Nationale 117, Lotissement Induslacq, 64170 Lacq, France
§ Université Paris 13, 74 rue Marcel Cachin, 93017 Bobigny, France
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00206
*E-mail: gerard-cahiez@chimie-paristech.fr.
//////////////http://pubs.acs.org/doi/suppl/10.1021/acs.oprd.7b00206/suppl_file/op7b00206_si_001.pdf



http://www.rsc.org/suppdata/c6/nj/c6nj03712g/c6nj03712g1.pdf