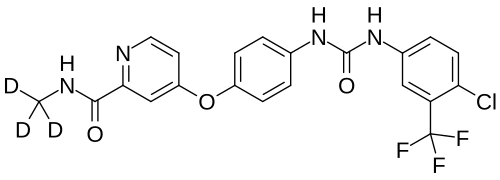
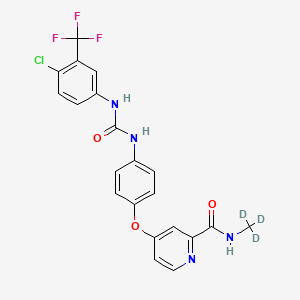
Donafenib
CAS 1130115-44-4, CM-4307, Zepsun, 41XGO0VS1U
4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-(trideuteriomethyl)pyridine-2-carboxamide
- 4-(4-(((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)amino)phenoxy)-N-(2H3)methylpyridine-2-carboxamide
- Sorafenib-d3
- Sorafenib D3
- Donafenib (Sorafenib D3)
- Sorafenib-methyl-d3
- d3-sorafenib
CM-4307 is under investigation in clinical trial NCT03602495 (Donafenib in 131I-Refractory Differentiated Thyroid Cancer).
Donafenib, sold under the brand name Zepsun, is a pharmaceutical drug for the treatment of cancer.
In China, donafenib is approved for the treatment of unresectable hepatocellular carcinoma in patients who have not previously received systemic treatment.[1][2]
Donafenib is a kinase inhibitor that targets Raf kinase and various receptor tyrosine kinases.[3] It is a deuterated derivative of sorafenib with improved pharmacokinetic properties.[4][5]
Donafenib is an orally available multikinase inhibitor that targets Raf kinase and various receptor tyrosine kinases (RTKs), with potential antineoplastic activity. Upon oral administration, donafenib binds to and blocks the activity of Raf kinase, and inhibits Raf-mediated signal transduction pathways. This inhibits cell proliferation in Raf-expressing tumor cells. In addition, this agent may inhibit unidentified RTKs, and thus may further block tumor cell proliferation in susceptible tumor cells. Raf, a serine/threonine protein kinase, plays a key role in the Raf/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathway. Deregulation of this pathway often results in tumor cell proliferation and survival.
SYN
ACS Omega 2021, 6, 5532−5547.
https://pubs.acs.org/doi/10.1021/acsomega.0c05908
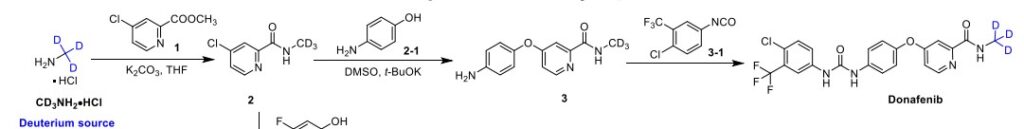
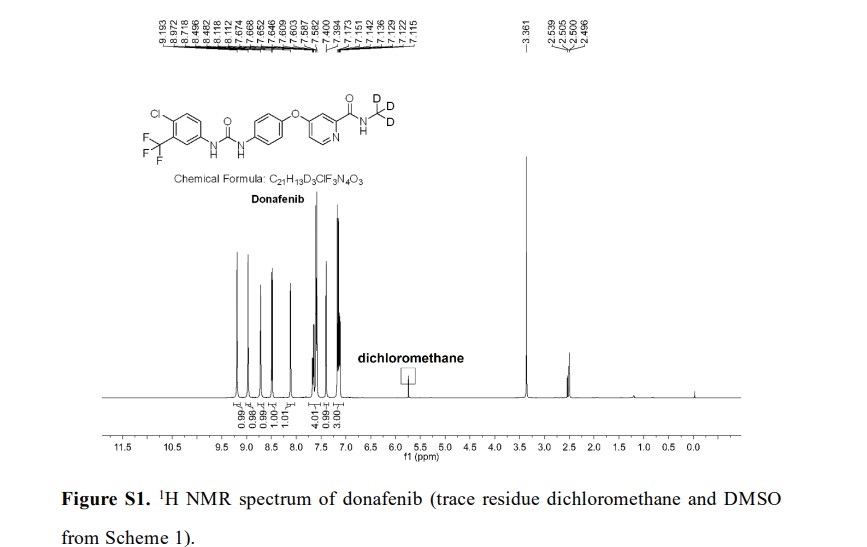
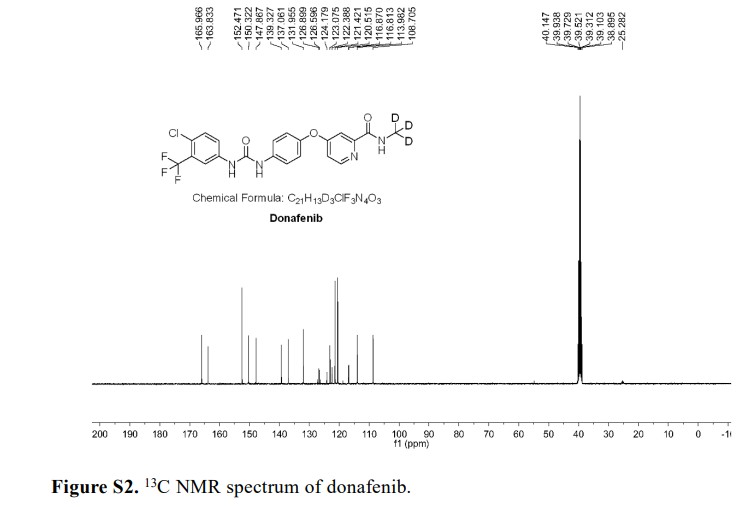
Syn
Donafenib (Zepsun). Donafenib (31), developed by Suzhou Zelgen Biopharmaceuticals, is a deuterated derivative of sorafenib, a multikinase inhibitor for the treatment of advanced hepatocellular carcinoma (HCC). 222 HCC is the most common type of primary liver cancer in adults and the third leading cause of cancer-related deaths worldwide.223,224 Donafenib inhibits Raf kinase and VEGFR tyrosine kinases,
thereby preventing the proliferation of tumor cells. 225 The presence of the deuterated methyl group in donafenib improves metabolic stability with prolonged half-life, lower systemic clearance, and higher systemic exposure.226 Donafenib has been shown to significantly improve the overall survival
of patients with HCC when compared against sorafenib, with favorable safety and tolerability.227 228
In June 2021, donafenib was first approved in China for treating unresectable HCC in patients who have not previously received systemic treatment.
A gram-scale synthesis of donafenib was recently disclosed by Luo and co-workers (Scheme 55).229
The synthetic sequence commenced with amidation of methyl ester 31.2 using methan-d3-amine hydrochloride (31.1) as the deuterium source, affording CD 3-amide 31.3 in high yield (98%). SNAr
displacement with aminophenol 31.4 in DMSO provided diaryl ether 31.5. Finally, reaction of the aniline moiety with isocyanate 31.6 delivered donafenib (31) in 79% yield from 31.3
(222) Mousa, A. B. Sorafenib in the treatment of advanced
hepatocellular carcinoma. Saudi J. Gastroenterol 2008, 14, 40−42.
(223) Forner, A.; Llovet, J. M.; Bruix, J. Hepatocellular carcinoma.
Lancet 2012, 379, 1245−1255.
(224) Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski,
A. Hepatocellular carcinoma. Lancet 2022, 400, 1345−1362.
(225) Gong, X.; Qin, S. Study progression of anti-angiogenetic
therapy and its combination with other agents for the treatment of
advanced hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2018, 7,
466−474.
(226) Zhong, L.; Hou, C.; Zhang, L.; Zhao, J.; Li, F.; Li, W.
Synthesis of deuterium-enriched sorafenib derivatives and evaluation
of their biological activities. Mol. Divers. 2019, 23, 341−350.
(227) Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu,
Y.; Meng, Z.; Pan, H.; et al. Donafenib versus sorafenib in first-line
treatment of unresectable or metastatic hepatocellular carcinoma: A
randomized, open-label, parallel-controlled phase II-III trial. J. Clin.
Oncol. 2021, 39, 3002−3011.
(228) Keam, S. J.; Duggan, S. Donafenib: First approval. Drugs 2021,
81, 1915−1920.
(229) Li, C.; Zhong, J.; Liu, B.; Yang, T.; Lv, B.; Luo, Y. Study on
typical diarylurea drugs or derivatives in cocrystallizing with strong H
bond acceptor DMSO. ACS Omega 2021, 6, 5532−5547.




AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Keam SJ, Duggan S (November 2021). “Donafenib: First Approval”. Drugs. 81 (16): 1915–1920. doi:10.1007/s40265-021-01603-0. PMID 34591285.
- Chen R, Ielasi L, di Carlo A, Tovoli F (February 2023). “Donafenib in hepatocellular carcinoma”. Drugs of Today. 59 (2): 83–90. doi:10.1358/dot.2023.59.2.3507751. hdl:11585/955557. PMID 36811408.
- “Donafenib”. NCI Cancer Dictionary. National Cancer Institute, National Institutes of Health.
- Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. (September 2021). “Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial”. Journal of Clinical Oncology. 39 (27): 3002–3011. doi:10.1200/JCO.21.00163. PMC 8445562. PMID 34185551.
- Qin S, Bi F, Xu J, Du C, Fan Q, Zhang L, et al. (2020). “P-86 Comparison of the pharmacokinetics of donafenib and sorafenib in patients with advanced hepatocellular carcinoma: An open-label, randomized, parallel-controlled, multicentre phase II/III trial”. Annals of Oncology. 31: S117 – S118. doi:10.1016/j.annonc.2020.04.168.
| Clinical data | |
|---|---|
| Trade names | Zepsun |
| Other names | CM-4307 |
| Legal status | |
| Legal status | Rx in China |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1130115-44-4 |
| PubChem CID | 25191001 |
| DrugBank | DB15414 |
| ChemSpider | 23937167 |
| UNII | 41XGO0VS1U |
| ChEMBL | ChEMBL4297490 |
| CompTox Dashboard (EPA) | DTXSID90648995 |
| Chemical and physical data | |
| Formula | C21H16ClD3F3N4O3 |
| Molar mass | 470.87 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
///////Donafenib, ZEPSUN, CHINA 2021, APPROVALS 2021, Suzhou Zelgen, 1130115-44-4, CM 4307, 41XGO0VS1U, Sorafenib D3














