_648927-86-0.png)
Firibastat
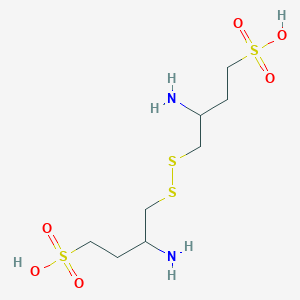
- Molecular FormulaC8H20N2O6S4
- Average mass368.514 Da
368.5
RB 150
Qgc-001(racemate)
UNII-PD5EII1F9A
Firibastat, (+/-)-
PD5EII1F9A
3-amino-4-[(2-amino-4-sulfobutyl)disulfanyl]butane-1-sulfonic acid
1-Butanesulfonic acid, 4,4′-dithiobis(3-amino-
3-Amino-4-((2-amino-4-sulfo-butyl)disulfanyl)butane-1-sulfonic acid
cas 721392-96-7, RACEMIC
CAS 648927-86-0, (S)-3-amino-4-(((S)-2-amino-4-sulfobutyl)disulfaneyl)butane-1-sulfonic acid
EXAMPLES
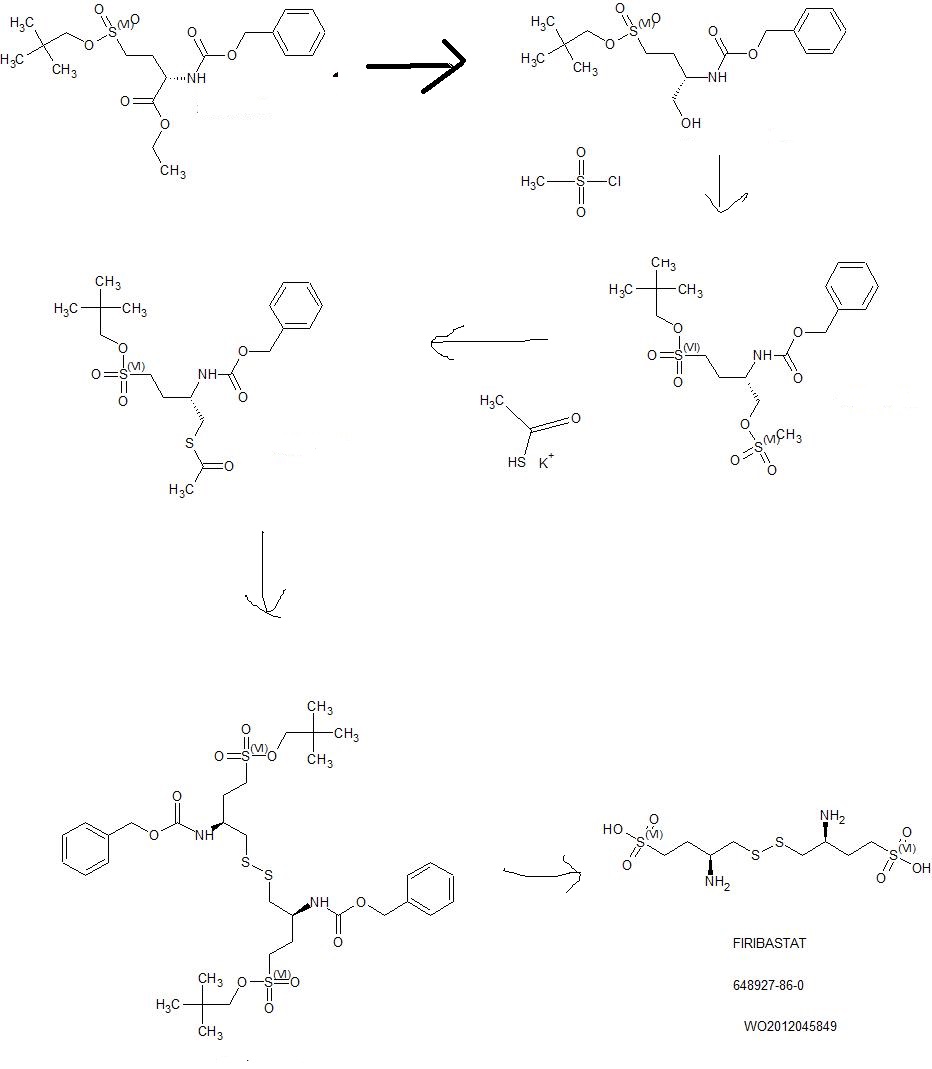
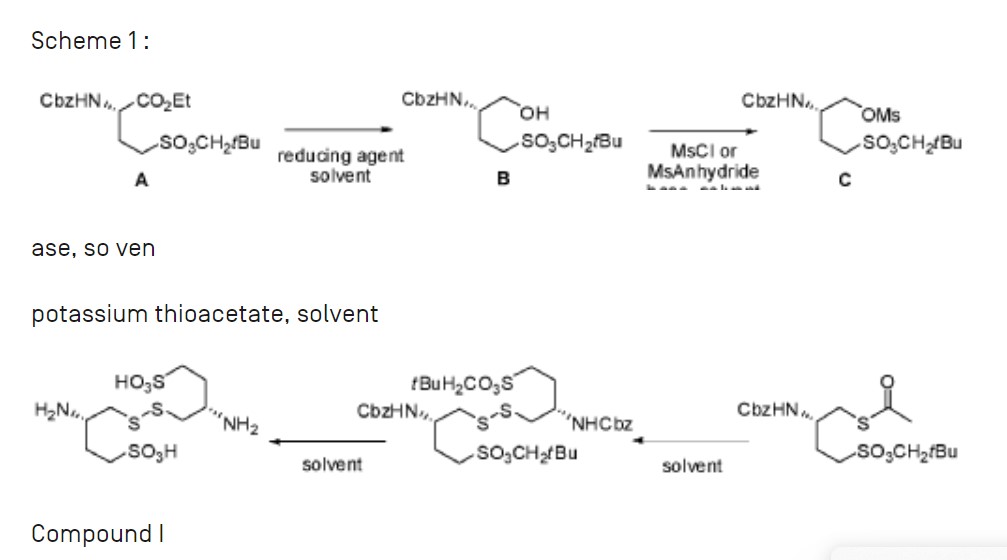
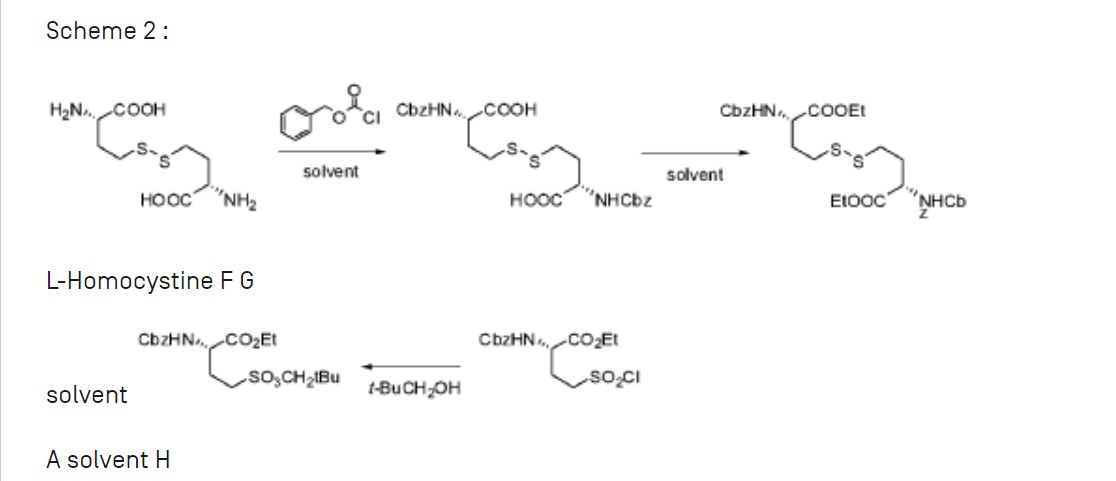
Example 1: Synthesis of compound I from (S) ethyl 2-(benzyloxycarbonylamino) 4-(neopentyloxysulfonyl)butanoate
Step (a): (S) neopentyl 3-(benzylox carbonylamino) 4-hydroxybutane 1-sulfonate B
B
(S) ethyl 2-(benzyloxycarbonylamino) 4-(neopentyloxysulfonyl)butanoate A (41.55g, 100.0 mmol, 1.0 eq.) is added dropwise onto a 2M solution of LiBH4 in THF (50 mL, 44.8 g, 100.0 mmol, 1.0 eq.). The addition is performed at room temperature over a 3 hrs period. At the end of the addition, the mixture is stirred at room temperature until conversion is complete (A<1%). Addition of toluene, followed by hydrolysis with HC1, washings of the organic layer with NaHC03 and water, and concentration under vacuum lead to the desired product as a pale yellow oil in quantitative yield (ee = 98%), which slowly crystallises at room temperature in 4 or 5 days.
As B was found to have a very low melting point by DSC analysis, it was not possible to isolate it as a solid by simple crystallisation. It was decided to let it in solution and use it without further purification in the following step.
Step (b): (S) neopentyl 3-(benzyloxycarbonylamino) 4-(methylsulfonyloxy)butane 1-sulfonate
C
C
A solution of B (57.64 g, 154.34 mmol, 1.0 eq.) in toluene (115 mL, 2.0 vol.) is diluted with MTBE (173 mL, 3.0 vol.) at room temperature. Mesyl chloride (17.9 mL, 26.5 g, 231.50 mmol, 1.5 eq.) is then added at room temperature and the homogeneous mixture is cooled to 10°C. The addition of triethylamine (43.0 mL, 31.2 g, 308.67 mmol, 2.0 eq.) is performed at T<20°C. At the end of the addition, the mixture is stirred at 10°C until conversion is complete (B<1%). After hydrolysis with diluted HCl, the organic layer is washed with NaHC03, water and brine, followed by a partial concentration under reduced pressure. The corresponding mesylate is then crystallised by addition of heptanes (5.0 vol.) at 40°C. After cooling, filtration and drying, the expected product is isolated as a whitish solid in 92.5% yield and with a very high chemical purity (98%).
Step (c): (S) 2-(benzyloxycarbonylamino) 4-(neopentyloxysulfonyl)butyl thioacetate D
D
A solution of mesylate C (81.3 g, 180.05 mmol, 1.0 eq.) in acetone (203 mL, 2.5 vol.) is added dropwise to a suspension of potassium thioacetate (41.1 g, 360.1 mmol, 2.0 eq.) in acetone (203 mL, 2.5 vol.) at room temperature and over a period of 2 hrs. The reaction mixture is stirred at room temperature until conversion is complete (C<1%). After filtration of the salts and addition of toluene (4.0 vol.), acetone is removed by distillation under reduced pressure at 25°C. The solution is then treated with active charcoal and concentrated to 2.0 volumes. Slow addition of heptane (5.0 vol.) at room temperature, followed by cooling at 0°C, filtration and drying at 45°C, provides the expected product as a whitish solid in 78.2% yield and with a very high chemical purity (98%).
Step (d): (3S,3S’) neopentyl 4,4′-disulfanediylbis(3-(benzyloxycarbonylamino)butane 1-sulfonate) E
E
A solution of D (59.16 g, 137.1 mmol, 1.0 eq.) suspended in ethanol (203 mL, 2.5 vol.) is cooled to 0°C. 20% sodium hydroxide (25.1 mL, 150.8 mmol, 1.1 eq.) diluted with water
(16.9 mL, 0.285 vol.) is then added dropwise to the suspension by keeping the temperature below 10°C. The reaction mixture is warmed to room temperature and stirred until conversion is complete (D<1%). The intermediate thiol reacts at room temperature with a solution of iodine (20.9 g, 82.3 mmol, 0.6 eq.) in ethanol (118 mL, 2.0 vol.). The reaction is complete at the end of the addition of the oxidizing agent. After addition of a Na2S205 (13.0 g, 68.5 mmol, 0.5 eq.) aqueous solution (118 mL, 2.0 vol.) to reduce the excess of residual iodine, ethanol is removed by distillation under reduced pressure at 40°C. Addition of water (3.0 vol.) at room temperature, followed by cooling at 0°C, filtration and drying at 45-50°C, provides the expected dimer as a white solid in 98.3% yield and with a very high chemical purity (97.0%). The amount of iodide ions, coming from the reduction of iodine, is checked in the sample by potentiometric assay.
E°(Ag+/Ag(s))=0.80V
KsAgi=1.5.10“16
[AgNO3]=0.1N
Electrode: E=E°(Ag+/Ag(s))+0.061og[Ag+]
E=E°(Ag+/Ag(s))+0.061og (Ksi/[L])
Assay: [T] decreases and E increases
LOD=l mg
Four further washings with water are performed until no more iodide ions are detected. The results are presented in table 2.
Table 2.
Step (e): (3S,3S’) 4,4′-disulfanediylbis(3-aminobutane 1-sulfonic acid) compound I
4
Compound I
A solution of E (44.0 g, 56.6 mmol, 1.0 eq.) in TFA (220 mL, 5.0 vol.) and anisole (44 mL, 1.0 vol.) is heated to reflux (75°C) and the reaction mixture is stirred in these conditions until conversion is complete (E<1%). TFA is removed by distillation under reduced pressure at 50°C. Slow addition of MTBE (5.0 vol.) at room temperature makes the expected product precipitate. After trituration, filtration and washing with MTBE (1.0 vol.), the crude solid is suspended in methanol (220 mL, 5.0 vol.). New trituration, filtration and washing with MTBE (1.0 vol.), followed by drying under reduced pressure, provides compound I as a white solid in 92.5% yield.
NMR: 1H (solvent D20, 400 MHz, ppm): 4.70 (s, 6H, ¾); 3.77 (m, 2H, H2); 3.14 (dd, 2H, Hi); 2.98 (dd, 4H, H4); 2.86 (dd, 2H, Hi); 2.13 (m, 4H, H3). 13C (solvent D20, 100 MHz, ppm): 49.4 (2C, C2); 46.6 (2C, C4); 38.3 (2C, C ; 26.9 (2C, C3).
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
- OriginatorCNRS; INSERM; University Paris Descartes
- DeveloperQuantum Genomics
- ClassAmines; Aminopeptidases; Antihypertensives; Cardiovascular therapies; Disulfides; Heart failure therapies; Metalloexopeptidases; Small molecules; Sulfonic acids
- Mechanism of ActionGlutamyl aminopeptidase inhibitors
- Orphan Drug StatusNo
- New Molecular EntityYes
- Phase IIIHypertension
- Phase IIChronic heart failure; Left ventricular dysfunction
- 28 Mar 2022No recent reports of development identified for phase-I development in Hypertension(In volunteers) in United Kingdom (PO, Tablet)
- 25 Nov 2021Firibastat licensed to Teva in Israel
- 11 Oct 2021Quantum Genomics plans a phase III trial for Heart failure
////////Firibastat, фирибастат , فيريباستات , firibastatum, фирибастат ,فيريباستات ,非立巴司他 , rb 150, (+/-)-QGC-001, qgc 001,
C(CS(=O)(=O)O)C(CSSCC(CCS(=O)(=O)O)N)N