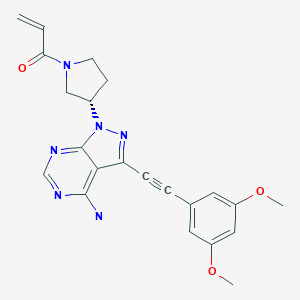
Futibatinib
フチバチニブ
| Formula |
C22H22N6O3
|
|---|---|
| CAS |
1448169-71-8
|
| Mol weight |
418.4485
|
2022/9/30 FDA APPROVED, Lytgobi
|
Antineoplastic, Receptor tyrosine kinase inhibitor
|
|
| Disease |
Cholangiocarcinoma (FGFR2 gene fusion)
|
|---|
1-[(3S)-3-[4-amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-pyrrolidinyl]-2-propen-1-one
TAS-120, TAS 120, TAS120; Futibatinib
Futibatinib, also known as TAS-120 is an orally bioavailable inhibitor of the fibroblast growth factor receptor (FGFR) with potential antineoplastic activity. FGFR inhibitor TAS-120 selectively and irreversibly binds to and inhibits FGFR, which may result in the inhibition of both the FGFR-mediated signal transduction pathway and tumor cell proliferation, and increased cell death in FGFR-overexpressing tumor cells. FGFR is a receptor tyrosine kinase essential to tumor cell proliferation, differentiation and survival and its expression is upregulated in many tumor cell types.
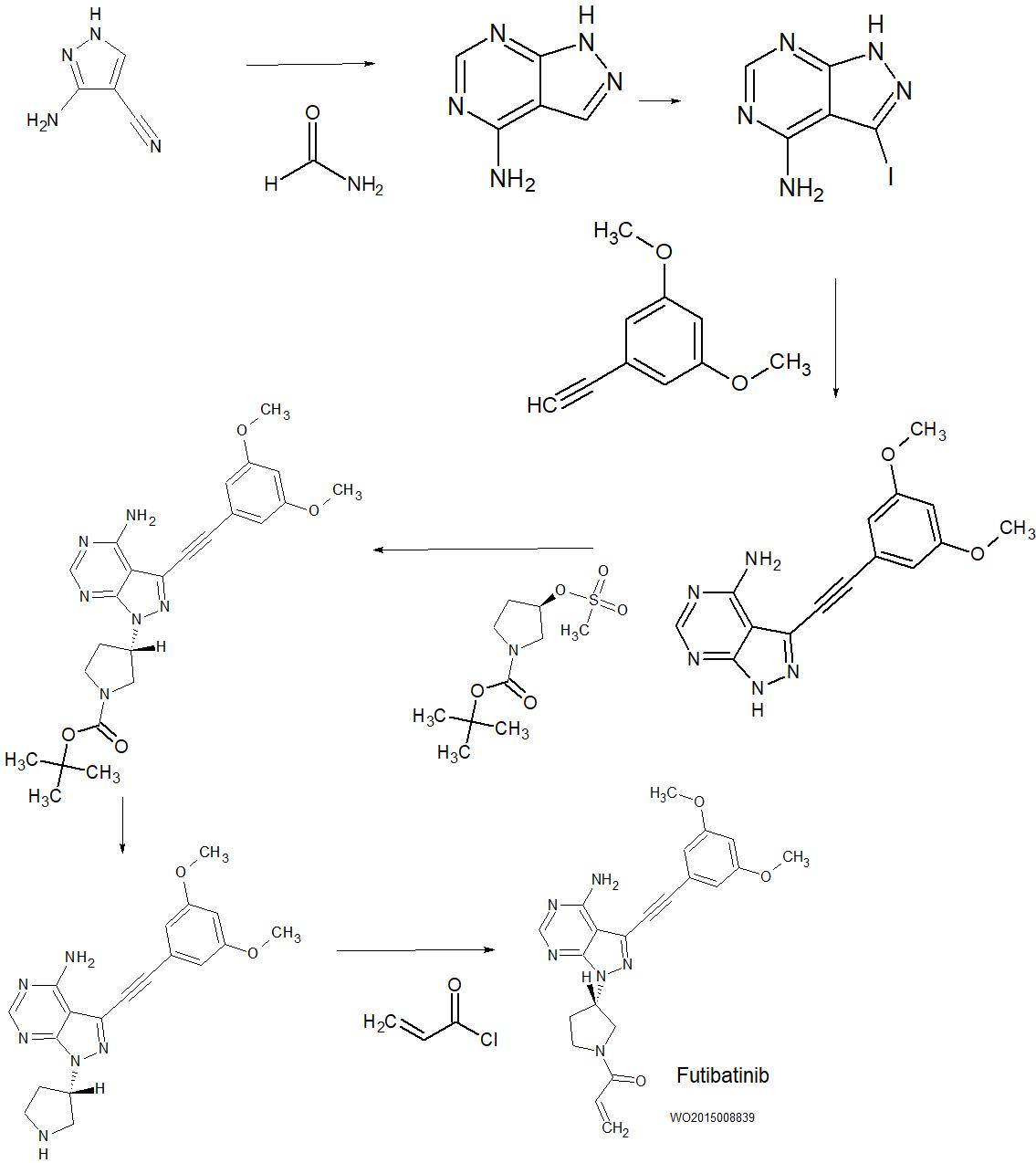
SYN
Patent Document 2: International Publication WO 2008/121742 pamphlet
Patent Document 3: International Publication WO 2010/043865 pamphlet
Patent Document 4: International Publication WO 2011/115937 pamphlet
Non-licensed Document 2: Mol. Cancer Res. 3, 655-667 (2005)
Non-licensed Document 3: Cancer Res. 70, 2085-2094 (2010)
Non-licensed Document 4: Clin. Cancer Res. 17, 6130-6139 (2011)
Non-licensed Document 5: Nat. Med. 1, 27-31 (1995)
WO2020095452
WO2020096042
WO2020096050
WO2019034075
WO2015008844
WO2015008839
WO2013108809
SYN
US9108973
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019034075&_cid=P10-L8Y7CQ-04809-1
Reference Example 1: WXR1
PAPER
Bioorg Med Chem, March 2013, Vol.21, No.5, pp.1180-1189
SYN
WO2015008844
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015008844&_cid=P10-L8Y448-45990-1
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020095452&_cid=P10-L8Y49P-48096-1
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
 |
|
| Clinical data | |
|---|---|
| Trade names | Lytgobi |
| Other names | TAS-120 |
| License data |
|
| Routes of administration |
By mouth |
| Drug class | Antineoplastic |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C22H22N6O3 |
| Molar mass | 418.457 g·mol−1 |
| 3D model (JSmol) | |
Futibatinib, sold under the brand name Lytgobi, is a medication used for the treatment of cholangiocarcinoma (bile duct cancer).[1][2] It is a kinase inhibitor.[1][3] It is taken by mouth.[1]
Futibatinib was approved for medical use in the United States in September 2022.[1][2][4]
Medical uses
Futibatinib is indicated for the treatment of adults with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.[1][2]
Names
Futibatinib is the international nonproprietary name (INN).[5]
References
- ^ Jump up to:a b c d e f “Lytgobi (futibatinib) tablets, for oral use” (PDF). Archived (PDF) from the original on 4 October 2022. Retrieved 4 October 2022.
- ^ Jump up to:a b c https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/214801Orig1s000ltr.pdf Archived 4 October 2022 at the Wayback Machine
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “Lytgobi (Futibatinib) FDA Approval History”. Archived from the original on 4 October 2022. Retrieved 4 October 2022.
- ^ “FDA Approves Taiho’s Lytgobi (futibatinib) Tablets for Previously Treated, Unresectable, Locally Advanced or Metastatic Intrahepatic Cholangiocarcinoma” (Press release). Taiho Oncology. 30 September 2022. Archived from the original on 4 October 2022. Retrieved 4 October 2022 – via PR Newswire.
- ^ World Health Organization (2019). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 81”. WHO Drug Information. 33 (1). hdl:10665/330896.
External links
- “Futibatinib”. Drug Information Portal. U.S. National Library of Medicine.
//////////Futibatinib, Lytgobi, FDA 2022, APPROVALS 2022, フチバチニブ , ANTINEOPLASTIC, TAS 120
C=CC(N1C[C@@H](N2N=C(C#CC3=CC(OC)=CC(OC)=C3)C4=C(N)N=CN=C42)CC1)=O