
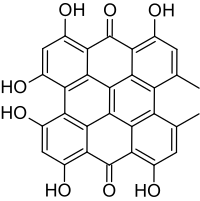
Hypericin
Hypericin is a naphthodianthrone, an anthraquinone derivative which, together with hyperforin, is one of the principal active constituents of Hypericum (Saint John’s wort).[2][3] Hypericin is believed to act as an antibiotic, antiviral[2] and non-specific kinaseinhibitor. Hypericin may inhibit the action of the enzyme dopamine β-hydroxylase, leading to increased dopamine levels, although thus possibly decreasing norepinephrine and epinephrine.
It was initially believed that the anti-depressant pharmacological activity of hypericin was due to inhibition of monoamine oxidase enzyme. The crude extract of Hypericum is a weak inhibitor of MAO-A and MAO-B. Isolated hypericin does not display this activity, but does have some affinity for NMDA receptors.[citation needed] This points in the direction that other constituents are responsible for the MAOI effect. The current belief is that the mechanism of antidepressant activity is due to the inhibition of reuptake of certain neurotransmitters.[2]
The large chromophore system in the molecule means that it can cause photosensitivity when ingested beyond threshold amounts.[citation needed] Photosensitivity is often seen in animals that have been allowed to graze on St. John’s Wort. Because hypericin accumulates preferentially in cancerous tissues, it is also used as an indicator of cancerous cells. In addition, hypericin is under research as an agent in photodynamic therapy, whereby a biochemical is absorbed by an organism to be later activated with spectrum-specific light from specialized lamps or laser sources, for therapeutic purposes. The antibacterial and antiviral effects of hypericin are also believed to arise from its ability for photo-oxidation of cells and viral particles.[2]
Hypericin derives from polyketides cyclisation.[4][5]
The biosynthesis of hypericins is in the polyketide pathway where an octaketide chain goes through processes of cylizations and decarboxylations form emodin anthrone which are believed to be the precursors of hypericin. Oxidization reactions yield protoforms which then are converted into hypericin and pseudohypericin. These reactions are photosensitive and take place under exposure to light and using the enzyme Hyp-1. [6][7][8][9][10]
References
- Jump up^ Merck Index, 11th Edition, 4799
- ^ Jump up to:a b c d Mehta, Sweety (2012-12-18). “Pharmacognosy of St. John’s Wort”. Pharmaxchange.info. Retrieved 2014-02-16.
- Jump up^ Oubre, Alondra (1991). “Hypericin: the active ingredient in Saint John’s Wort”. Archived from the original on September 28, 2007. Retrieved September 18, 2006.
- Jump up^ Loren W. Walker (1999). “A Review of the Hypothetical Biogenesis and Regulation of Hypericin synthesis via the Polyketide Pathway in Hypericum perforatum and Experimental Methods Proposed to Evaluate the Hypothesis”.
- Jump up^ Christian Hertweck (2009). “Polyketide Biosynthesis”. Angew. Chem. Int. Ed. 48: 4688–4716. doi:10.1002/anie.200806121.
- Jump up^ Karioti A, Bilia AR (2010) Hypericins as potential leads for new therapeutics. Int J Mol Sci 11:562-594
- Jump up^ Falk H (1999) From the photosensitizer hypericin to the photoreceptorstentorian—the chemistry of phenanthroperylene quinines. AngewChem Int Ed 38:3116–3136
- Jump up^ Bais HP, Vepachedu R, Lawrence CB, Stermitz FR, Vivanco JM (2003)Molecular and biochemical characterization of an enzyme responsible for the formation of hypericin in St. John’s wort(Hypericum perforatum L.). J Biol Chem 278:32413–32422
- Jump up^ Michalska K, Fernades H, Sikorski M, Jaskolski M (2010) Crystal structure of Hyp-1, a St. John’s wort protein implicated in the biosynthesis of hypericin. J Struct Biol 169:161–171
- Jump up^ Murthy, Hosakatte Niranjana et al. “Hypericins: Biotechnological Production from Cell and Organ Cultures.” Applied Microbiology and Biotechnology 98.22 (2014): 9187–9198. PubMed. Web.
An Efficient Multigram Synthesis of Hypericin Improved by a Low Power LED Based Photoreactor
http://pubs.acs.org/doi/10.1021/acs.oprd.7b00317

- Maringá, Brazil
- Professor
Abstract
In this work, an improved synthesis process was developed for the multigram production of hypericin. An inexpensive and efficient low power Light Emission Diode (LED) based photoreactor was designed and employed to perform the protohypericin photocyclization reaction allowing its photoconversion in hypericin. This closed system overcomes safety issues related to scale-up hypericin preparation typically described in the literature which combines the use of open systems, organic solvents, and high-power light sources. The photoreactor designed allows a solution to, mainly, the intrinsic effect of hypericin photobleaching inherent to the protohypericin photocyclization reaction, implying an increase in the yield of the final product and consequently the final cost. Using a red-LED based photoreactor, a safety protocol was carried out in a 5-g scale hypericin preparation with quantitative yield.
C30H16O8;
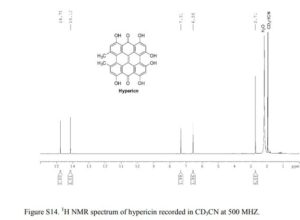
1H NMR (CD3CN, 25 °C, 500 MHz): δH 14.75 (s, 2H, OH-1, OH-6), 14.13 (s, 2H, OH-7, OH-12), 7.31 (s, 2H, Ar-H8, Ar-H11), 6.55 (s, 2H, Ar-H2, Ar-H5), 2.71 (s, 6H, Ar-CH3) ppm; UV–vis (EtOH) λmax 392, 480, 513, 552, 596.
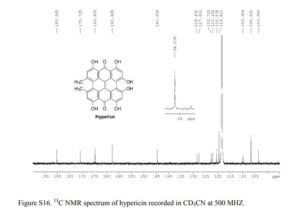
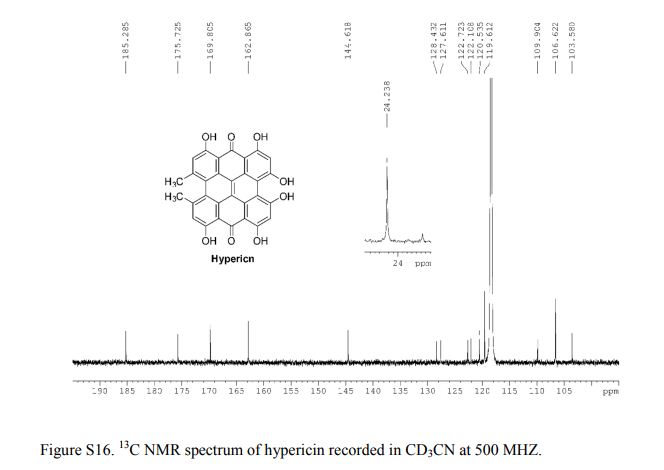
13C NMR (CD3CN, 25 °C, 500 MHz): δC 185.2, 175.7, 169.8, 162.8, 144.6, 128.4, 127.6, 122.7, 122.1, 120.5, 119.6, 109.9, 106.6, 103.5.
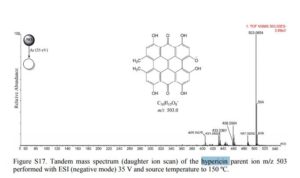
The identification of molecular formula of Hypericin (C30H16O8) was confirmed on its negative HRMS ion of m/z 503.0854 (calcd for [M – H]− 503.0845). The MS2 experiment showed fragment ions characteristic for this compound of m/z 487.0202 [M–H–CH4]−, 458.0504 [M–H–CO2–H·]−, 433.0361 [M–H–CH2═C═O–CO]−, 431.0601 [M–H–CO2–CO]−, and 405.0425 [M–H–CH2═C═O–2CO]−.
The characterization data of hypericin are in agreement with the literature.(35-39)
- 35.
Kapinus, E. I.; Falk, H.; Tran, H. T. N. Monatsh. Chem. 1999, 130, 623– 635 DOI: 10.1007/s007060050222
- 36.
Piperopoulos, G.; Lotz, R.; Wixforth, A.; Schmierer, T.; Zeller, K.-P. J. Chromatogr., Biomed. Appl. 1997, 695, 309– 316 DOI: 10.1016/S0378-4347(97)00188-6
- 37.
Brolis, M.; Gabetta, B.; Fuzzati, N.; Pace, R.; Panzeri, F.; Peterlongo, F. J. Chromatogr. A 1998, 825, 9– 16 DOI: 10.1016/S0021-9673(98)00697-9
- 38.
Riedel, K.-D.; Rieger, K.; Martin-Facklam, M.; Mikus, G.; Haefeli, W. E.; Burhenne, J. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2004,813, 27– 33 DOI: 10.1016/j.jchromb.2004.09.061
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[1,10,9,8-opqra]perylene-7,14-dione
|
|
| Other names
4,5,7,4′,5′,7′-Hexahydroxy-2,2′-dimethylnaphthodianthrone
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.129 |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| C30H16O8 | |
| Molar mass | 504.45 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
/////////////Hypericin