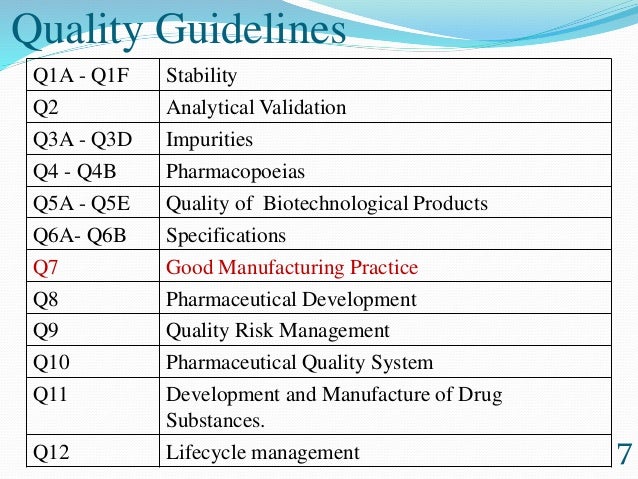
ICH Q11 Q and A Document
The topic of starting materials has been a vexed topic for some period. Indeed concerns relating to lack of clarity and issues pertaining to practical implementation led the EMA in Sept 2014 to publish a reflection paper—Reflection on the requirements for selection and justification of starting materials for the manufacture of chemical active substances.(10) The paper sought to outline key issues as well as authority expectations; specific areas of interest identified included the following:
| 1. |
Variance in interpretation between applicant and reviewer. |
||||
| 2. |
The registration of short syntheses that employ complex custom-made starting materials. |
||||
| 3. |
Lack of details preventing authorities being able to assess the suitability of a proposed registered starting material and its associated control strategy. |
||||

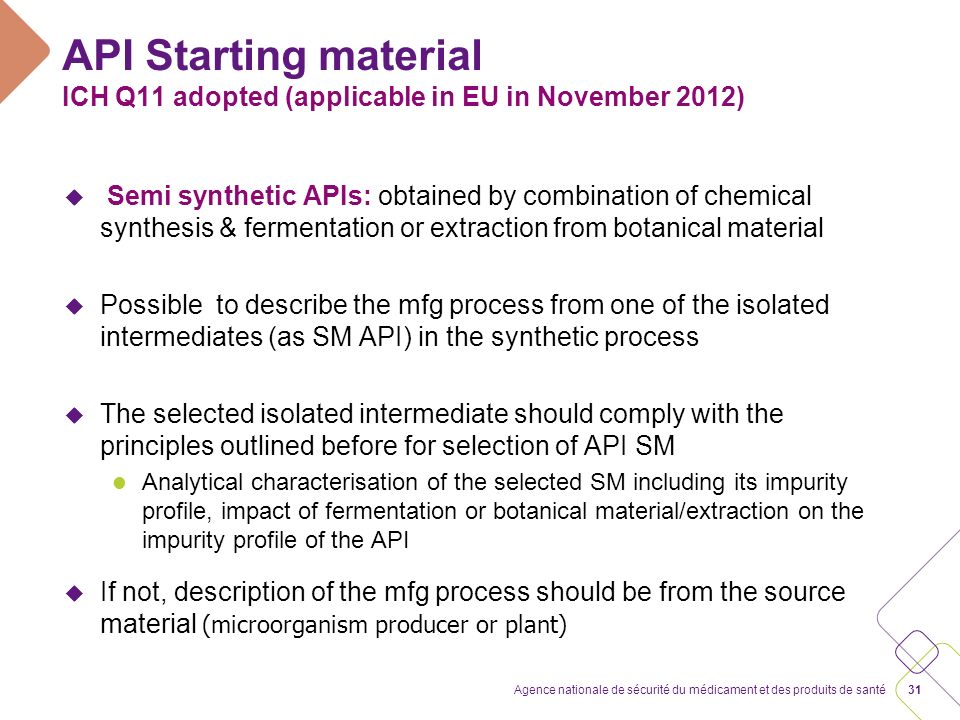
The questions and answers are aligned to specific sections within the guideline, although all questions are focused specifically on Section 5—Selection of starting material and source material. There are 16 questions in total covering the following aspects:

| 1. |
Significant Structural Fragment: how should this be interpreted |
||||
| 2. |
Impact on Impurity profile of final product: this relates to the guidance within Q11 to include all stages that impact on impurity profile of the drug substance. This seeks to clarify what level would be defined as impactful. |
||||
| 3. |
Clarification of persistence |
||||
| 4. |
How an applicant should determine which steps impact the profile of mutagenic impurities in the drug substance. |
||||
| 5. |
Do all steps that involve mutagenic reagents, impurities, or establish regio- or stereochemical configurations, need to be included in the process description? |
||||
| 6. |
Clarification of the stated need to describe “enough” of the drug substance manufacturing |
||||
| 7. |
Should all the ICH Q11 general principles be considered and met in selecting starting materials? |
||||
| 8. |
Application of Q11 principles to telescoped processes |
||||
| 9. |
Application of Q11 to linear and convergent syntheses |
||||
| 10. |
Starting material specifications: key attributes |
||||
| 11. |
Noncommercial starting materials |
||||
| 12. |
Differences: Commercially available vs Custom Synthesis |
||||
| 13. |
Requirements for justification of commercial availability |
||||
| 14. |
Scope: postapproval change–preregistered starting materials |
||||
| 15. |
Life cycle management |
||||
| 16. |
Starting material Q11 vs Q7 definition: clarification that this is effectively the same |
||||
Key points of note include
-
“Significant structural fragment”: the document highlights the frequent misconception that this means that a proposed starting material should be structurally similar to the drug substance. It makes clear that in fact the intent is simply to help distinguish between reagents, catalysts, solvents, or other raw materials (which do not contribute a “significant structural fragment” to the molecular structure of the drug substance) from materials that do.
-
Questions 5.2, 5.4, and 5.5 relate to mutagenic impurities. The answers provided should be useful in assisting an applicant in applying the risk based approach defined within ICH M7.(5)Prior to this there was a general misconception that a step that involved a mutagenic impurity needed to be part of the registered process. Such an assertion took no account of the highly reactive nature of such impurities and their propensity to be effectively purged.(12) The answer to question 5.2 makes clear the framework for defining the impact of an MI on the quality of the final drug substance, aligning this directly to M7 and the adoption of the widely applied 30% of the limit principle, i.e. prove levels in the final active drug substance are below 30% of the acceptable limit. The answer to question 5.4. provides a commentary of the actual practical steps involved in assessing the impact of an MI. Importantly within this it contextualizes the actual risk posed by low level MIs with the following important statement
-
“Such mutagenic impurities and by-products are usually present at much lower concentrations than reagents, solvents, and intermediates. Therefore, the risk that such impurities will carry over significantly into the drug substance from early reaction steps is lower than for reagents, solvents, or intermediates from the same steps.”
-
In essence, provided any MI associated with a starting material is demonstrably controlled it is not necessary to register stages that simply employ the use of mutagenic reagents.
-
Another important point addressed within the document is ‘persistency.’ It seeks to make clear that even where an impurity associated with a starting material does impact on the quality of the drug substance that control can be defined at the stage of the starting material. A classic example would be a stereoisomer. General downstream processing would have little impact on levels. In many cases this has led to a view that the step where such an impurity might arise must form part of the registered process, i.e. the stage of introduction of chirality. This now makes clear that, provided this is effectively controlled within the starting material, registration of earlier stages is not required.
The response highlights several key aspects
Another key point made is the need for an applicant to examine steps immediately upstream of those identified as critical and within those upstream to consider if:
-
They include a unit operation that has been added to the manufacturing process to control specific impurities that would otherwise impact the impurity profile of the drug substance.
The key point made here is that you cannot simply add multiple purification steps prior to a proposed starting material.
-
Tight control (e.g., within narrow parameter ranges) is required to prevent generation of impurities that would otherwise impact the impurity profile of the drug substance.
Perhaps the most contentious aspect of the response though is the caveat that if having conducted the assessment described and if based on this the result is that only a small number of chemical transformation steps need to be registered, the Q&A document articulates a need to include one or more additional steps. The reasons stated for this needing to be considered are
-
Due to the risk of contamination arising from a late starting material and the impact this would have on drug substance quality and
-
The risk of changes made to the route/process for the starting material impacting again on drug substance quality.
References
-
1.Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities EMA/CHMP/ CVMP/ SWP/169430/ 2012,http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/11/WC500177735.pdf.
-
2.ICH Q11 – Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities)Q11http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf.
-
3.Teasdale, A.Regulatory Highlights Org. Process Res. Dev. 2015, 19 ( 4) 494– 498, DOI: 10.1021/acs.oprd.5b00085
-
4.ICH Q3D Guideline for Elemental Impuritieshttp://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step_4.pdf.
-
5.Assessment and Control of Dna Reactive (Mutagenic)Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7(R1) March 2017,http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M7/M7_R1_Addendum_Step_4_31Mar2017.pdf.
-
6.ICH Q3A Impurities in New Drug Substanceshttp://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3A_R2/Step4/Q3A_R2__Guideline.pdf.
-
7.ICH Q3B Impurities in New Drug Productshttp://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3B_R2/Step4/Q3B_R2__Guideline.pdf.
-
8.Harvey, J.; Teasdale, A.; Fleetwood, A.Management of organic impurities in small molecule medicinal products: Deriving safe limits for use in early development Regul. Toxicol. Pharmacol. 2017, 84, 116– 123,DOI: 10.1016/j.yrtph.2016.12.011
-
9.Questions and answers on implementation of risk based prevention of cross contamination in production and ‘Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities’ (EMA/CHMP/CVMP/SWP/169430/ 2012) .http://www.ema.europa.eu/docs/en_GB/document_library/Other/2017/01/WC500219500.pdf.
-
10.Reflection paper on the requirements for selection and justification of starting materials for the manufacture of chemical active substanceshttp://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500175228.pdf.
-
11.ICH guideline Q11 on development and manufacture of drug substances (chemical entities and biotechnological/biological entities) – questions and answershttp://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Q_A_Step_2.pdf.
-
12.Teasdale, A.Risk Assessment of Genotoxic Impurities in New Chemical Entities: Strategies to Demonstrate Control Org. Process Res. Dev. 2013, 17, 221– 230, DOI: 10.1021/op300268u
-
13.EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Usehttps://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/chapter_5.pdf.
///////////ICH Q11, Q and A Document















