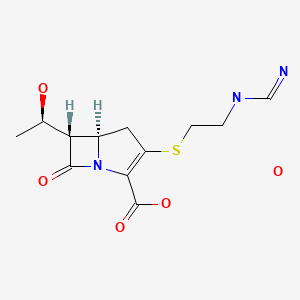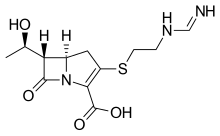
Imipenem
イミペネム水和物
Cas 74431-23-5
- Molecular FormulaC12H19N3O5S
- Average mass317.361 Da
(5R,6S)-3-((2-(Form
Antibacterial, Cell wall biosynthesis inhibitor
Imipenem (Primaxin among others) is an intravenous β-lactam antibiotic discovered by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s.[1] Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria,[2] thus play a key role in the treatment of infections not readily treated with other antibiotics.[3]
Imipenem was patented in 1975 and approved for medical use in 1985.[4] It was discovered via a lengthy trial-and-error search for a more stable version of the natural product thienamycin, which is produced by the bacterium Streptomyces cattleya. Thienamycin has antibacterial activity, but is unstable in aqueous solution, so impractical to administer to patients.[5] Imipenem has a broad spectrum of activity against aerobic and anaerobic, Gram-positive and Gram-negative bacteria.[6] It is particularly important for its activity against Pseudomonas aeruginosa and the Enterococcus species. It is not active against MRSA, however.
Medical uses
Spectrum of bacterial susceptibility and resistance
Acinetobacter anitratus, Acinetobacter calcoaceticus, Actinomyces odontolyticus, Aeromonas hydrophila, Bacteroides distasonis, Bacteroides uniformis, and Clostridium perfringens are generally susceptible to imipenem, while Acinetobacter baumannii, some Acinetobacter spp., Bacteroides fragilis, and Enterococcus faecalis have developed resistance to imipenem to varying degrees. Not many species are resistant to imipenem except Pseudomonas aeruginosa (Oman) and Stenotrophomonas maltophilia.[7]
Coadministration with cilastatin
Imipenem is rapidly degraded by the renal enzyme dehydropeptidase 1 when administered alone, and is almost always coadministered with cilastatin to prevent this inactivation[8]
Adverse effects
Common adverse drug reactions are nausea and vomiting. People who are allergic to penicillin and other β-lactam antibiotics should take caution if taking imipenem, as cross-reactivity rates are high. At high doses, imipenem is seizurogenic.[9]
Mechanism of action
Imipenem acts as an antimicrobial through inhibiting cell wall synthesis of various Gram-positive and Gram-negative bacteria. It remains very stable in the presence of β-lactamase (both penicillinase and cephalosporinase) produced by some bacteria, and is a strong inhibitor of β-lactamases from some Gram-negative bacteria that are resistant to most β-lactam antibiotics.
SYM
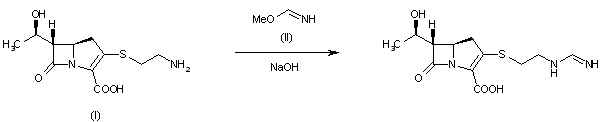
By reaction of thienamycin (I) with methyl formimidate (II) by means of NaOH in water.
| DE 2652679; FR 2332012; GB 1570990; NL 7612939 |
SYN 2
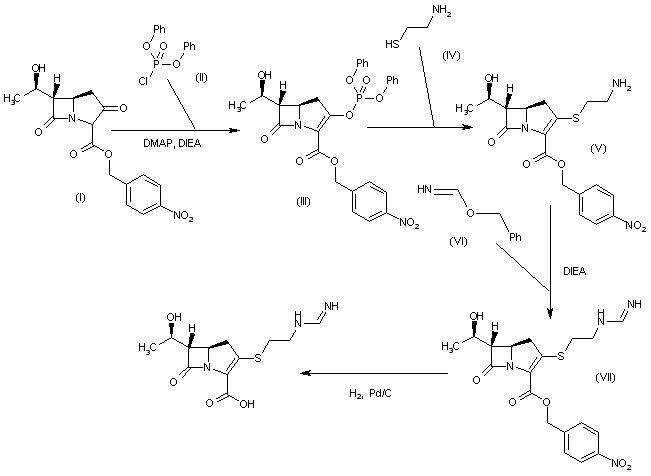
WO 0294828
The reaction of (3R,5R,6S)-6-(1(R)-hydroxyethyl)-2-oxo-1-carbapenem-3-carboxylic acid p-nitrobenzyl ester (I) with diphenyl chlorophosphate by (II) means of DMAP and DIEA in DMA/dichloromethane gives the enol phosphate (III), which is condensed with 2-aminoethanethiol (IV) in DMA to yield the 2-aminoethylsulfanyl derivative (V). The reaction of (V) with benzyl formimidate (VI) by means of DIEA in DMA affords the intermediate p-nitrobenzyl ester (VII), which is finally hydrogenated with H2 over Pd/C in water/isopropanol/N-methylmorpholine to provide the target Imipemide.
SYN 3
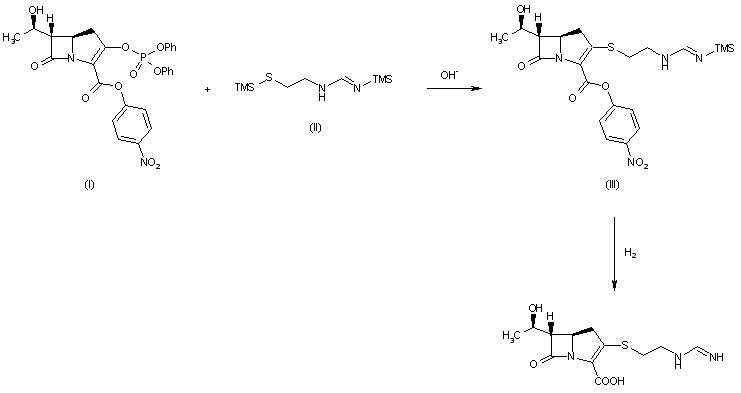
Tetrahedron Lett 1982,23(47),4903
The condensation of 7-oxo-6-(1-hydroxyethyl)-3-(diphenoxyphosphate)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid p-nitrophenyl ester (I) with the bis(trimethylsilyl) derivative of 2-(iminomethylamino)ethanethiol (II) in the presence of base gives p-nitrophenyl ester of MK-0787, protected with a trimethylsilyl group (III), which is finally deprotected by hydrogenolysis.
CLIP

Synthesis Path
References
- ^ U.S. Patent 4,194,047
- ^ Clissold, SP; Todd, PA; Campoli-Richards, DM (Mar 1987). “Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy”. Drugs. 33 (3): 183–241. doi:10.2165/00003495-198733030-00001. PMID 3552595.
- ^ Vardakas, KZ; Tansarli, GS; Rafailidis, PI; Falagas, ME (Dec 2012). “Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis”. The Journal of Antimicrobial Chemotherapy. 67 (12): 2793–803. doi:10.1093/jac/dks301. PMID 22915465.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 497. ISBN 9783527607495.
- ^ Kahan, FM; Kropp, H; Sundelof, JG; Birnbaum, J (Dec 1983). “Thienamycin: development of imipenen-cilastatin”. The Journal of Antimicrobial Chemotherapy. 12 Suppl D: 1–35. doi:10.1093/jac/12.suppl_d.1. PMID 6365872.
- ^ Kesado, Tadataka; Hashizume, Terutaka; Asahi, Yoshinari (1980). “Antibacterial activities of a new stabilized thienamycin, N-formimidoyl thienamycin, in comparison with other antibiotics”. Antimicrobial Agents and Chemotherapy. 17 (6): 912–7. doi:10.1128/aac.17.6.912. PMC 283902. PMID 6931548.
- ^ “Imipenem spectrum of bacterial susceptibility and Resistance” (PDF). Retrieved 4 May 2012.
- ^ “IMIPENEM/CILASTATIN”. livertox.nih.gov. Retrieved 2019-03-08.
- ^ Cannon, Joan P.; Lee, Todd A.; Clark, Nina M.; Setlak, Paul; Grim, Shellee A. (2014-08-01). “The risk of seizures among the carbapenems: a meta-analysis”. Journal of Antimicrobial Chemotherapy. 69 (8): 2043–2055. doi:10.1093/jac/dku111. ISSN 0305-7453.
Further reading
- Clissold, SP; Todd, PA; Campoli-Richards, DM (1987). “Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy”. Drugs. 33(3): 183–241. doi:10.2165/00003495-198733030-00001. PMID 3552595.
- Buckley, MM; Brogden, RN; Barradell, LB; Goa, KL (1992). “Imipenem/cilastatin. A reappraisal of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy”. Drugs. 44 (3): 408–44. doi:10.2165/00003495-199244030-00008. PMID 1382937.
External links
- Imipenem bound to proteins in the PDB
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Primaxin |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a686013 |
| Pregnancy category |
|
| Routes of administration |
IM, IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 20% |
| Metabolism | Renal |
| Elimination half-life | 38 minutes (children), 60 minutes (adults) |
| Excretion | Urine (70%) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.058.831 |
| Chemical and physical data | |
| Formula | C12H17N3O4S |
| Molar mass | 299.347 g/mol g·mol−1 |
| 3D model (JSmol) | |
- Synonyms:Imipemide
- ATC:J01DH51
- Use:carbapenem antibiotic
- Chemical name:[5R-[5α,6α(R*)]]-6-(1-hydroxyethyl)-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
- Formula:C12H17N3O4S
- MW:299.35 g/mol
- CAS-RN:64221-86-9
- InChI Key:ZSKVGTPCRGIANV-ZXFLCMHBSA-N
- InChI:InChI=1S/C12H17N3O4S/c1-6(16)9-7-4-8(20-3-2-14-5-13)10(12(18)19)15(7)11(9)17/h5-7,9,16H,2-4H2,1H3,(H2,13,14)(H,18,19)/t6-,7-,9-/m1/s1
- EINECS:264-734-5
- LD50:1660 mg/kg (M, i.v.); >5 g/kg (M, p.o.);
1972 mg/kg (R, i.v.); >5 g/kg (R, p.o.)
Derivatives, monohydrate
- Formula:C12H17N3O4S • H2O
- MW:317.37 g/mol
- CAS-RN:74431-23-5
References
-
-
Leanza, W.J. et al.: J. Med. Chem. (JMCMAR) 22, 1435 (1979).
-
a Salzmann, T.L. et al.: J. Am. Chem. Soc. (JACSAT) 102, 6161-6163 (1980).
-
Reider, P.J.; Grabowski, E.J.J.: Tetrahedron Lett. (TELEAY) 23, 2293-2296 (1982).
-
Grabowski, E.J.J.: Chirality (CHRLEP) 17, 249-259 (2005).
-
US 4 194 047 (Merck & Co.; 18.3.1980; prior. 21.11.1975).
-
DOS 2 652 679 (Merck & Co.; appl. 19.11.1976; USA-prior. 21.11.1975).
-
b US 5 998 612 (Merck & Co.; 7.12.1999; appl. 12.6.1992; prior. 23.10.1981).
-
c US 4 981 992 (Takasago; 27.1.1998; appl. 13.5.1996; J-prior. 11.5.1995).
-
US 5 204 460 (Takasago; 20.4.1993; appl. 8.11.1991; J-prior. 8.11.1990).
-
US 5 204 462 (Takasago; 20.4.1993; appl. 8.11.1991; J-prior. 8.11.1990).
-
US 5 712 388 (Takasago; 27.1.1998; appl. 13.5.1996; J-prior. 11.5.1995).
-
US 5 081 239 (Takasago; 14.1.1992; appl. 29.11.1989; J-prior. 29.11.1988).
-
-
Acetoxylation of 2-azetidinones in 4-position:
-
Noyori, R. et al.: J. Am. Chem. Soc. (JACSAT) 111, 9134-9135 (1989).
-
Noyori, R. et al.: Angew. Chem. (ANCEAD) 114, 2108-2123 (2002).
-
US 5 288 862 (Takasago; 22.2.1994; appl. 16.4.1992; J-prior. 18.4.1991).
-
US 5 606 052 (Takasago; 25.2.1997; appl. 16.4.1992; J-prior. 18.4.1991).
-
-
Noyori-catalyst:
-
US 4 739 084 (Takasago; 19.4.1988; appl. 15.4.1987; J-prior. 13.5.1986).
-
-
d process of Nippon Soda (Nisso):
-
US 5 026 844 (Suntory & Nippon Soda; 25.6.1991; appl. 13.10.1989; J-prior. 19.10.1988).
-
US 5 792 861 (Tanabe Seiyaku & Nippon Soda; 11.8.1998; appl. 29.6.1994, 4.11.1996; J-prior. 30.6.1993).
-
US 5 808 055 (Suntory & Nippon Soda; 15.9.1998; appl. 30.3.1993, 5.7.1995; J-prior. 30.3.1993).
-
e US 4 791 198 (Kanegafuchi; 13.12.1988; appl. 1.7.1985, 6.1.1987; J-prior. 5.7.1984, 14.1.1986).
-
US 4 861 877 (Kanegafuchi; 29.8.1989; appl. 1.7.1985, 6.1.1987; J-prior. 5.7.1984, 14.1.1985, 14.1.1986).
-
US 5 061 817 (Kanegafuchi; 29.10.1991; appl. 1.7.1985, 6.1.1987, 31.5.1988; J-prior. 5.7.1984, 14.1.1986).
-
US 4 914 200 (Kanegafuchi; 3.4.1990; appl. 28.4.1987, 14.2.1989; J-prior. 30.4.1986, 13.11.1986, 9.2.1987).
-
-
Enzymatic reduction of alkyl-2-(N-benzoylamino)methyl-3-oxobutyrates with bakers yeast:
-
US 5 463 047 (Ciba-Geigy; 31.10.1995; appl. 15.9.1994; CH-prior. 4.5.1987).
-
-
Further synthesis processes of Merck & Co. for thienamycin:
-
Johnston, D.B.R. et al.: J. Am. Chem. Soc. (JACSAT) 100, 313-315 (1978).
-
Mellilo, D.G. et al.: Tetrahedron Lett. (TELEAY) 21, 2783 (1980).
-
Melillo, D.G. et al.: J. Org. Chem. (JOCEAH) 51, 1498-1504 (1986).
-
Karady, S. et al.: J. Am. Chem. Soc. (JACSAT) 103, 6765-6767 (1981).
-
US 4 269 772 (Merck & Co.; 26.5.1981; appl. 14.1.1980).
-
US 4 282 148 (Merck & Co.; 4.8.1981; appl. 14.1.1980).
-
US 4 287 123 (Merck & Co.; 1.9.1981; appl. 14.1.1980).
-
US 4 290 947 (Merck & Co.; 22.9.1981; appl. 29.5.1980).
-
US 4 360 684 (Merck & Co.; 23.11.1982; appl. 8.4.1981).
-
US 4 206 219 (Merck & Co.; 3.6.1980; appl. 24.10.1978).
-
US 4 348 320 (Merck & Co.; 7.9.1982; appl. 20.8.1980; USA-prior. 19.11.1976).
-
US 4 460 507 (Merck & Co.; 17.7.1984; appl. 29.4.1982; USA-prior. 10.10.1980).
-
US 5 055 573 (Merck & Co.; 8.10.1991, appl. 24.8.1990; USA-prior. 19.11.1976).
-
US 5 037 974 (Merck & Co.; 6.8.1991; appl. 14.8.1990; prior. 23.5.1988, 10.4.1990).
-
-
Review of thienamycin syntheses:
-
Nicolaou, K.C.; Sorensen, E.J.: Classics in Total Synthesis, VCH 1996, Weinheim & New York, chapter 16, p. 249-263.
-
Berks, A.H.: Tetrahedron (TETRAB) 52, 331-375 (1996).
-
-
Alternative 2-azetidinone ring closure with chlorosulfonyl isocyanate:
-
US 4 350 631 (Merck & Co.; 21.9.1982; appl. 18.3.1981; prior. 18.12.1980).
-
-
Thienamycin (by fermentation of S. cattleya):
-
US 3 950 357 (Merck & Co.; 13.4.1976; appl. 25.11.1974).
-
DOS 2 552 638 (Merck & Co.; appl. 24.11.1975; USA-prior. 25.11.1974).
-
-
Combination with cilastatin:
-
EP 48 301 (Merck & Co.; appl. 24.9.1980).
-
/////////////Imipenem, イミペネム水和物 , MK-787,