IOHEXOL
CAS Registry Number: 66108-95-0
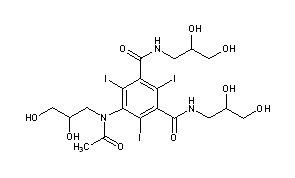
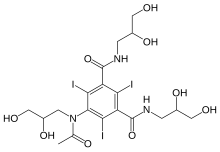
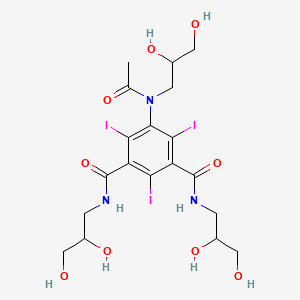
N1,N3-bis(2,3-dihydroxypropyl)-5-[N-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodobenzene-1,3-dicarboxamide
CAS Name: 5-[Acetyl(2,3-dihydroxypropyl)amino]-N,N¢-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Additional Names: N,N¢-bis(2,3-dihydroxypropyl)-5-[N-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodoisophthalamide
Manufacturers’ Codes: Win-39424; Compd 545
Trademarks: Omnipaque (GE Healthcare)
Molecular Formula: C19H26I3N3O9
Molecular Weight: 821.14
Percent Composition: C 27.79%, H 3.19%, I 46.36%, N 5.12%, O 17.54%
Literature References: Nonionic radio-contrast medium. Prepn: V. Nordal, H. Holtermann, DE 2726196; eidem, US 4250113 (1977, 1981 both to Nyegaard). HPLC-UV determn in plasma: R. S. Soman et al., J. Chromatogr. B 816, 339 (2005).
Pharmacology and toxicology: Acta Radiol. Suppl. 362, 1-134 (1980). Acute toxicity: S. Salvesen, ibid. 73. Fibrillatory potential in dogs: G. L. Wolf et al., Invest. Radiol. 16, 320 (1981).
Comparative clinical studies in coronary angiography: G. B. J. Mancini et al., Am. J. Cardiol. 51, 1218 (1983); I. D. Sullivan et al., Br. Heart J. 51, 643 (1984); M. A. Bettmann et al., Radiology 153, 583 (1984). Review: T. Almén, Acta Radiol. Suppl. 366, 9-19 (1983).
Properties: Crystals from butanol, mp 174-180°. Sol in water. Stable in aqueous solutions. Viscosity (cP): 6.2 at 37°; 12.6 at 20° (c = 200 mg Iodine/ml). LD50 in male, female rats, mice (g Iodine/kg): 15.0, 12.3, 24.3, 25.1 i.v. (Salvesen).
Melting point: mp 174-180°
Toxicity data: LD50 in male, female rats, mice (g Iodine/kg): 15.0, 12.3, 24.3, 25.1 i.v. (Salvesen)
Therap-Cat: Diagnostic aid (radiopaque medium).
Keywords: Diagnostic Aid (Radiopaque Medium).
Synthesis ReferenceXiu C. Wang, Steve A. Chamberlin, Ashok V. Bhatia, Gregg E. Robinson, John Hufnagel, “Process for the preparation of iohexol.” U.S. Patent US5705692, issued December, 1985.
US5705692
Iohexol, sold under the trade name Omnipaque among others, is a contrast agent used for X-ray imaging.[1] This includes when visualizing arteries, veins, ventricles of the brain, the urinary system, and joints, as well as during computed tomography (CT scan).[1] It is given by mouth, injection into a vein, or into a body cavity.[2]
Iohexol is a contrast agent for intrathecal administration used in myelography and contrast enhancement for computerized tomography.
Side effects include vomiting, skin flushing, headache, itchiness, kidney problems, and low blood pressure.[1] Less commonly allergic reactions or seizures may occur.[1] Allergies to povidone-iodine or shellfish do not affect the risk of side effects more than other allergies.[3] Use in the later part of pregnancy may cause hypothyroidism in the baby.[4] Iohexol is an iodinated non-ionic radiocontrast agent.[1] It is in the low osmolar family.[5]
Iohexol was approved for medical use in 1985.[6] It is on the World Health Organization’s List of Essential Medicines.[7][2]
Chemistry
The osmolality of iohexol ranges from 322 mOsm/kg—approximately 1.1 times that of blood plasma—to 844 mOsm/kg, almost three times that of blood.[8] Despite this difference, iohexol is still considered a low-osmolality contrast agent; the osmolality of older agents, such as diatrizoate, may be more than twice as high.[9]
Society and culture
Names
It is sold under the brand names Omnipaque[10] and Hexopaque. It is also sold as a density gradient medium under the names Accudenz, Histodenz and Nycodenz.[11][12]
Formulations
It is available in various concentrations, from 140[citation needed] to 350[13] milligrams of iodine per milliliter.
PATENT
https://patents.google.com/patent/WO2005003080A1/en#:~:text=Primary%20production%20of%20iohexol%20involves,and%20a%20thorough%20purification%20stage.&text=The%20solvent%20is%20then%20evaporated,and%20recrystallised%20twice%20from%20butanol.
The present invention relates to a process for the manufacture of iohexol, 5-[N- (2,3- dihydroxypropyl) -acetamido]-N,N’-bis(2,3 -dihydroxypropyl)-2,4,6- triiodoisophtalamide.
Iohexol is the non-proprietory name of the chemical drug substance of a non-ionic iodinated X-ray contrast agent marketed under the trade name OMNIPAQUE®. OMNIPAQUE® is one of the most used agents in diagnostic X-ray procedures.
The manufacture of such non-ionic contrast agents involves the production of the chemical drug substance (referred to as primary production) followed by formulation into the drug product (referred to as secondary production). Primary production of iohexol involves a multistep chemical synthesis and a thorough purification stage. For a commercial drug product it is important for the primary production to be efficient and economical and to provide a drug substance fulfilling the specifications.
The final step in the synthesis of iohexol is a N-alkylation step in which 5-
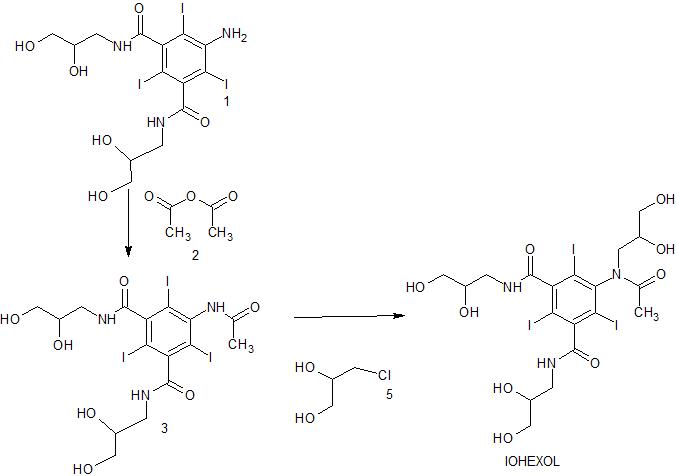
(acetamido)-N,N’-bis(2,3-dihydroxypropyl)-2,4,6 triiodoisophtalamide (hereinafter 5- Acetamide) is reacted in the liquid phase with an alkylating agent to introduce the 2,3-dihydroxypropyl group at the nitrogen of the 5-acetamido group. Following this reaction, iohexol is isolated from the reaction mixture and purified by crystallisation and treatment with ion exchange resins.
The manufacture of iohexol is disclosed for example in US-4,250,113 which is hereby incorporated by reference. In the last step of the multistep chemical synthesis crude iohexol is obtained from the reaction between 5-Acetamide and 1-chloro-2,3- propandiol at ambient temperature in propylene glycoi and in the presence of sodium methoxide. The solvent is then evaporated and crude iohexol is obtained. The crude product is evaporated to dryness and recrystallised twice from butanol.
Several suggestions to improve the N-alkylation and the purification steps have been published. WO-A-98/08804 discloses the use of 2-methoxy-ethanol and optionally isopropanol both in the alkylation step of 5-Acetamide and in the purification of crude iohexol. WO-A-02/083623 discloses the purification of crude iohexol using 1- methoxy-2-propanol as the solvent optionally in a mixture with other solvents.
The N-alkylation step where 5-Acetamide in solution is reacted with an alkylation agent such as e.g. 1-chloro-2,3-propandiol to introduce the 2,3-dihydroxypropyl group at the nitrogen of the 5-acetamido group is illustrated in Scheme 1 :
5-acatamido-N,N’-bis(2,3-dihydroxypropyl)- 5-[N-(2,3-dihydroxypropyl)acetamido]- 2,4,6-triiodoisophtalamide N,N’-bis(2,3-dihydroxypropyl)- 2,4,6-triiodoisophtalamide
Scheme 1.
The N-alkylation step is challenging because O-alkylated by-products can also be formed when the alkylation occurs at the oxygen atoms of the hydroxy groups. It is therefore a desire to limit the formation of these O-alkylated by-products and thereby to limit their presence in the final purified iohexol. The upper limit for values for O- alkylated by-products in the end product is fixed by the European Pharmacopea to 0.6% (HPLC by area).
The O-alkylated by-products are removed to the degree desired or necessary by recrystallisation steps. Further unidentified by-products also referred to as impurities are also formed during the alkylation reaction and must be reduced to a tolerable level. In addition the solvents used should be easily available, be environmentally friendly and be of low toxicity.
There is therefore a need to identify a solvent that can be used in the N-alkylation reaction and that fulfil the desiderata mentioned above. It is further desired to improve the overall process including the N-alkylation step and the purification step in the manufacture of iohexol. If the crude product obtained by the N-alkylation step is to be re-crystallised from a solvent that is different from the solvent used in the N- alkylation step, then the reaction solvent must first be removed e.g. by evaporation to dryness. It is known from crystallisation theory and experience that even small quantities of residual solvents from previous steps may cause a crystallisation process to get out of control due to changes in its supersaturation conditions, and thorough removal of the reaction solvent is an important step. Solvent removal is an energy consuming operation which also risks degradation of the product due to exposure to elevated temperature.
Example 1 : Synthesis of iohexol in 1-methoxy-2-propanol/methanol
1-methoxy-2-propanol (44 ml), methanol (19 ml) and sodium hydroxide (4.87 g) was added to a jacketed glass reactor and stirred for about 15 minutes at 25°C. 5-Acetamide (70 g) was added to the reactor, and the mixture stirred overnight at 45°C, before it was allowed to cool to 25°C. 1-chloro-2,3-propanediol (12.43 g) was added to the solution. After 1.5 hours, more 1-chloro-2,3-propanediol (0.83 g) was added, and the reaction was allowed to proceed for 24 hours. HPLC analysis (water/acetonitrile) of the reaction mixture gave the following results:
Iohexol 98.1 %
5-Acetamide 1.17 % O-alkylated substances 0.58 %
Other impurities 0.1 %
Example 2: Synthesis of iohexol in 1 -methoxy-2-propanol/water
1-methoxy-2-propanol (63 ml), water (7 ml) and sodium hydroxide (4.50 g) was added to a jacketed glass reactor and stirred for about 15 minutes at 25°C. 5-Acetamide (70 g) was added to the reactor, and the mixture stirred overnight at 45°C, before it was allowed to cool to 35°C. 1-chloro-2,3-propanediol (11.39 g) was added to the solution. After 3 hours, more 1-chloro-2,3-propanediol (0.83 g) was added, and the reaction was allowed to proceed for 24 hours. HPLC analysis (water/acetonitrile) of the reaction mixture gave the following results:
Iohexol 98.3 % 5-Acetamide 0.68 %
O-alkylated substances 0.81 %
Other impurities 0.3 % Example 3: Alkylation and crystallisation in solutions containing 1-methoxy-2- propanol
1-methoxy-2-propanol (63 L), methanol (27 L) and sodium hydroxide (6.96 kg) was added to a 500 L reactor and stirred until all solids were dissolved and the temperature was below 30°C. 5-Acetamide (100 kg) was added to the reactor, and the mixture stirred overnight at 45°C before it was allowed to cool to 25°C. 1-chloro- 2,3-propanediol (16.76 kg) was added to the clear solution. After 1.5 hours, more 1- chloro-2,3-propanediol (1.18 kg) was added, and the reaction was allowed to proceed for 30 hours. HPLC analysis (water/acetonitrile) of the reaction mixture gave the following results:
Iohexol 97.9 % 5-Acetamide 0.9 %
O-alkylated substances 0.83 %
Other impurities 0.4 %
The reaction was stopped by addition of hydrochloric acid (650 ml), and the reaction mixture diluted with a mixture of 1-methoxy-2-propanol (53 L) and methanol (13 L). The mixture was filtered, and the salts on the filter washed with methanol (3×10 L). The combined filtrate and wash was diluted with water (22 L) and treated with cationic ion exchange resin (AMB 200C, 80 L) and anionic ion exchange resin (IRA 67, 80 L) to a salt content of 0.006 w/w %. The solution was filtered, and the ion exchange resins washed in several stages with a mixture of water (160 L) and methanol (85 L). The combined filtrate and wash was concentrated under reduced pressure to a volume of 155 L. One half of this was taken further to crystallisation as described below.
Water was removed from the solution by azeotropic distillation. The volume was held at a constant level by replacing the distillate by 1-methoxy-2-propanol (80 L). At water content of 0.16 Ukg iohexol, further 1-methoxy-2-propanol (159 L) was added, and the solution seeded with iohexol crystals (0.26 kg). After stirring at reflux overnight, the volume of the solution was reduced by 42 L by distillation under reduced pressure (300-600 mbar). The temperature was set to 90°C, which was held for 3 hours before cooling to 60°C over 3 hours. The crystallisation mixture was stirred overnight at 60°C, filtered and washed with isopropanol (90 L, 6 portions). The yield was 48.4 kg (as dry powder), corresponding to 88-weight % corrected for seeding material and samples. HPLC analysis (water/acetonitrile) of the crystals gave the following results:
Iohexol 99.3 %
5-Acetamide 0.15 %
O-alkylated substances 0.45 %
Other impurities 0.11 %
PAPER
PATENT
The present invention relates to a process for the manufacture of iohexol, 5-[N- (2,3- dihydroxypropyl) -acetamido]-N,N’-bis(2,3 -dihydroxypropyl)-2,4,6- triiodoisophtalamide.
Iohexol is the non-proprietory name of the chemical drug substance of a non-ionic iodinated X-ray contrast agent marketed under the trade name OMNIPAQUE®. OMNIPAQUE® is one of the most used agents in diagnostic X-ray procedures.
The manufacture of such non-ionic contrast agents involves the production of the chemical drug substance (referred to as primary production) followed by formulation into the drug product (referred to as secondary production). Primary production of iohexol involves a multistep chemical synthesis and a thorough purification stage. For a commercial drug product it is important for the primary production to be efficient and economical and to provide a drug substance fulfilling the specifications.
The final step in the synthesis of iohexol is a N-alkylation step in which 5-
(acetamido)-N,N’-bis(2,3-dihydroxypropyl)-2,4,6 triiodoisophtalamide (hereinafter 5- Acetamide) is reacted in the liquid phase with an alkylating agent to introduce the 2,3-dihydroxypropyl group at the nitrogen of the 5-acetamido group. Following this reaction, iohexol is isolated from the reaction mixture and purified by crystallisation and treatment with ion exchange resins.
The manufacture of iohexol is disclosed for example in US-4,250,113 which is hereby incorporated by reference. In the last step of the multistep chemical synthesis crude iohexol is obtained from the reaction between 5-Acetamide and 1-chloro-2,3- propandiol at ambient temperature in propylene glycoi and in the presence of sodium methoxide. The solvent is then evaporated and crude iohexol is obtained. The crude product is evaporated to dryness and recrystallised twice from butanol.
Several suggestions to improve the N-alkylation and the purification steps have been published. WO-A-98/08804 discloses the use of 2-methoxy-ethanol and optionally isopropanol both in the alkylation step of 5-Acetamide and in the purification of crude iohexol. WO-A-02/083623 discloses the purification of crude iohexol using 1- methoxy-2-propanol as the solvent optionally in a mixture with other solvents.
The N-alkylation step where 5-Acetamide in solution is reacted with an alkylation agent such as e.g. 1-chloro-2,3-propandiol to introduce the 2,3-dihydroxypropyl group at the nitrogen of the 5-acetamido group is illustrated in Scheme 1 :
5-acatamido-N,N’-bis(2,3-dihydroxypropyl)- 5-[N-(2,3-dihydroxypropyl)acetamido]- 2,4,6-triiodoisophtalamide N,N’-bis(2,3-dihydroxypropyl)- 2,4,6-triiodoisophtalamide
Scheme 1.
The N-alkylation step is challenging because O-alkylated by-products can also be formed when the alkylation occurs at the oxygen atoms of the hydroxy groups. It is therefore a desire to limit the formation of these O-alkylated by-products and thereby to limit their presence in the final purified iohexol. The upper limit for values for O- alkylated by-products in the end product is fixed by the European Pharmacopea to 0.6% (HPLC by area).
The O-alkylated by-products are removed to the degree desired or necessary by recrystallisation steps. Further unidentified by-products also referred to as impurities are also formed during the alkylation reaction and must be reduced to a tolerable level. In addition the solvents used should be easily available, be environmentally friendly and be of low toxicity.
There is therefore a need to identify a solvent that can be used in the N-alkylation reaction and that fulfil the desiderata mentioned above. It is further desired to improve the overall process including the N-alkylation step and the purification step in the manufacture of iohexol. If the crude product obtained by the N-alkylation step is to be re-crystallised from a solvent that is different from the solvent used in the N- alkylation step, then the reaction solvent must first be removed e.g. by evaporation to dryness. It is known from crystallisation theory and experience that even small quantities of residual solvents from previous steps may cause a crystallisation process to get out of control due to changes in its supersaturation conditions, and thorough removal of the reaction solvent is an important step. Solvent removal is an energy consuming operation which also risks degradation of the product due to exposure to elevated temperature.
Example 1 : Synthesis of iohexol in 1-methoxy-2-propanol/methanol
1-methoxy-2-propanol (44 ml), methanol (19 ml) and sodium hydroxide (4.87 g) was added to a jacketed glass reactor and stirred for about 15 minutes at 25°C. 5-Acetamide (70 g) was added to the reactor, and the mixture stirred overnight at 45°C, before it was allowed to cool to 25°C. 1-chloro-2,3-propanediol (12.43 g) was added to the solution. After 1.5 hours, more 1-chloro-2,3-propanediol (0.83 g) was added, and the reaction was allowed to proceed for 24 hours. HPLC analysis (water/acetonitrile) of the reaction mixture gave the following results:
Iohexol 98.1 %
5-Acetamide 1.17 % O-alkylated substances 0.58 %
Other impurities 0.1 %
Example 2: Synthesis of iohexol in 1 -methoxy-2-propanol/water
1-methoxy-2-propanol (63 ml), water (7 ml) and sodium hydroxide (4.50 g) was added to a jacketed glass reactor and stirred for about 15 minutes at 25°C. 5-Acetamide (70 g) was added to the reactor, and the mixture stirred overnight at 45°C, before it was allowed to cool to 35°C. 1-chloro-2,3-propanediol (11.39 g) was added to the solution. After 3 hours, more 1-chloro-2,3-propanediol (0.83 g) was added, and the reaction was allowed to proceed for 24 hours. HPLC analysis (water/acetonitrile) of the reaction mixture gave the following results:
Iohexol 98.3 % 5-Acetamide 0.68 %
O-alkylated substances 0.81 %
Other impurities 0.3 % Example 3: Alkylation and crystallisation in solutions containing 1-methoxy-2- propanol
1-methoxy-2-propanol (63 L), methanol (27 L) and sodium hydroxide (6.96 kg) was added to a 500 L reactor and stirred until all solids were dissolved and the temperature was below 30°C. 5-Acetamide (100 kg) was added to the reactor, and the mixture stirred overnight at 45°C before it was allowed to cool to 25°C. 1-chloro- 2,3-propanediol (16.76 kg) was added to the clear solution. After 1.5 hours, more 1- chloro-2,3-propanediol (1.18 kg) was added, and the reaction was allowed to proceed for 30 hours. HPLC analysis (water/acetonitrile) of the reaction mixture gave the following results:
Iohexol 97.9 % 5-Acetamide 0.9 %
O-alkylated substances 0.83 %
Other impurities 0.4 %
The reaction was stopped by addition of hydrochloric acid (650 ml), and the reaction mixture diluted with a mixture of 1-methoxy-2-propanol (53 L) and methanol (13 L). The mixture was filtered, and the salts on the filter washed with methanol (3×10 L). The combined filtrate and wash was diluted with water (22 L) and treated with cationic ion exchange resin (AMB 200C, 80 L) and anionic ion exchange resin (IRA 67, 80 L) to a salt content of 0.006 w/w %. The solution was filtered, and the ion exchange resins washed in several stages with a mixture of water (160 L) and methanol (85 L). The combined filtrate and wash was concentrated under reduced pressure to a volume of 155 L. One half of this was taken further to crystallisation as described below.
Water was removed from the solution by azeotropic distillation. The volume was held at a constant level by replacing the distillate by 1-methoxy-2-propanol (80 L). At water content of 0.16 Ukg iohexol, further 1-methoxy-2-propanol (159 L) was added, and the solution seeded with iohexol crystals (0.26 kg). After stirring at reflux overnight, the volume of the solution was reduced by 42 L by distillation under reduced pressure (300-600 mbar). The temperature was set to 90°C, which was held for 3 hours before cooling to 60°C over 3 hours. The crystallisation mixture was stirred overnight at 60°C, filtered and washed with isopropanol (90 L, 6 portions). The yield was 48.4 kg (as dry powder), corresponding to 88-weight % corrected for seeding material and samples. HPLC analysis (water/acetonitrile) of the crystals gave the following results:
Iohexol 99.3 %
5-Acetamide 0.15 %
O-alkylated substances 0.45 %
Other impurities 0.11 %
Patent
CN109134289
N-Acylation of 5-amino-N,N’-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (1) with acetic anhydride (2) in the presence of p-TsOH gives 5-(acetylamino)-N,N’-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (3) , which upon condensation with glycidol using NaOMe in 2-methoxyethanol at 90 °C or epichlorohydrin by means of NaHCO3 in propylene glycol at 85 °C or 3-chloropropane-1,2-diol (5) using aqueous NaOH furnishes the iohexol .
(7) synthesis of Iodixanol
Modus ponens (I) compound (200g, 0.28mol) be added 1L there-necked flask in, thereto be added acetic anhydride (207g, 2.03mol), acetic acid (103.3mL), p-methyl benzenesulfonic acid monohydrate (1g, 5.42mmol), finishes reaction solution being heated to 60 DEG C Start to react, keep the temperature 30 minutes after reacting liquid temperature reaches 120-125 DEG C, cooling is concentrated into after can just stirring thereto It is added 50%v/v (600mL), is slowly added dropwise thereto into 50%w/v sodium hydrate aqueous solution, by adding in reaction process The mode of 50%w/v sodium hydrate aqueous solution keeps the pH of reaction solution between 11~12, and reaction temperature is maintained at 40-45 DEG C, Reaction is finished, and concentrated hydrochloric acid is added into reaction solution and adjusts pH3-4, and stirring filters after 3.0 hours, and filter cake is washed with water to neutrality, dries It is dry, obtain white solid 187g, yield 88.2%, HPLC98.14%.
Go step obtained solid (150g, 0.2mol) be added there-necked flask in, thereto be added sodium hydroxide (14.4g, 0.36mol), purified water (300mL), epoxychloropropane (27.9g, 0.30mol) finish 30-35 DEG C of reaction 72.0 hours, instead It should finish, adjust pH3-4, Iodixanol HPLC purity 72.5%, Iohexol HPLC11.3% with concentrated hydrochloric acid.
(4) synthesis of Iohexol
Modus ponens (I) compound (200g, 0.28mol) be added 1L there-necked flask in, thereto be added acetic anhydride (432g, 4.23mol) flows back 3.0 hours, be then concentrated under reduced pressure into p-methyl benzenesulfonic acid monohydrate (1g, 5.42mmol), agitating and heating It can just stir, be added portionwise into reaction solution methanol (25g), methanol is added after 1.0 hours in stirring thereto again (140g) is finished and is stirred to react 1.0 hours, and being concentrated under reduced pressure into can just stir, and purified water (20g) then is added thereto, 60 DEG C are finished to be stirred overnight.
Reaction solution is cooled to 30 DEG C hereinafter, extracting reaction solution 200mL, stirring is lower will with 50%w/v sodium hydrate aqueous solution Reaction solution pH is adjusted to 12, the addition 1- chloro- 2 into reaction solution, 3-propanediol (20g, 0.18mol), passes through benefit in reaction process The mode of 50%w/v sodium hydrate aqueous solution is added to keep the pH of reaction solution between 11~12, after reaction 12.0 hours thereto Add 1- chloro- 2,3-propanediol (3g, 29.29mmol) finishes that the reaction was continued 48.0 hours, and reaction solution samples HPLC detection, iodine Mykol purity is 89.9%.
(5) synthesis of Ioversol
Modus ponens (I) compound (200g, 0.28mol) is added in 1L there-necked flask, and N-Methyl pyrrolidone is added thereto Chloracetyl chloride (200mL) is added in (200mL) thereto under stirring, finish 50-53 DEG C and react 3.0 hours, and reaction is finished, and is cooled to 20 DEG C, reaction solution is slowly added in methanol (2000mL).It finishing, flows back 9.0 hours, reaction is finished, and is cooled to 25 DEG C, it filters, Filter cake is washed with methanol, and drying obtains white solid 177g, yield 79.8%, HPLC purity 98.3%.
It takes previous step obtained solid (150g, 0.19mol) to be added in 1L there-necked flask, purified water 300mL is added thereto, Acetic acid sodium trihydrate (183g, 1.34mol) finishes back flow reaction, by adding 50%w/v sodium hydroxide water in reaction process The mode of solution keeps the pH of reaction solution between 5-6, and reaction is finished, and concentrated hydrochloric acid is added into reaction solution, adjusts pH3-4, stirring It being filtered after 3.0 hours, filter cake is with purifying water washing to neutrality, and drying obtains white solid 127g, yield 86.7%, HPLC98.4%.
It takes step obtained solid (100g, 0.13mol), is added in 1L there-necked flask, purified water 300mL, chlorine are added thereto Change sodium (46.5g, 0.796mol), finish, be warming up to 50 DEG C, 10N sodium hydrate aqueous solution (39.3mL) and 2- are added thereto Chlorethanol (63.5g, 0.79mol) finishes 48-52 DEG C of heat preservation and reacts 5.0 hours, and reaction is finished, and concentrated hydrochloric acid is added thereto and adjusts PH6.5, reaction solution HPLC detection, Iohexol purity 89.7%.
(6) synthesis of Iopentol
Modus ponens (I) compound (200g, 0.28mol) be added 1L there-necked flask in, thereto be added acetic anhydride (432g, 4.23mol) flows back 3.0 hours, be then concentrated under reduced pressure into p-methyl benzenesulfonic acid monohydrate (1g, 5.42mmol), agitating and heating It can just stir, be added portionwise into reaction solution methanol (25g), methanol (140g) is added thereto again after stirring 1.0 hours, It finishes and is stirred to react 1.0 hours, being concentrated under reduced pressure into can just stir, and purified water (20g) then is added thereto, finishes 60 DEG C It is stirred overnight.
Reaction solution is cooled to 30 DEG C hereinafter, extracting reaction solution 200mL, stirring is lower will with 50%w/v sodium hydrate aqueous solution Reaction solution pH is adjusted to 12, and the chloro- 3- methoxy-2-propanol (22.5g, 0.18mol) of 1-, reaction process are added into reaction solution In keep the pH of reaction solution between 11~12 by way of adding 50%w/v sodium hydrate aqueous solution, react 12.0 hours Add 1- chloro- 2 thereto afterwards, 3-propanediol (3.4g, 29.29mmol) finishes that the reaction was continued 48.0 hours, reaction solution sampling HPLC detection, Iopentol purity are 91.3%.
(7) synthesis of Iodixanol
Modus ponens (I) compound (200g, 0.28mol) be added 1L there-necked flask in, thereto be added acetic anhydride (207g, 2.03mol), acetic acid (103.3mL), p-methyl benzenesulfonic acid monohydrate (1g, 5.42mmol), finishes reaction solution being heated to 60 DEG C Start to react, keep the temperature 30 minutes after reacting liquid temperature reaches 120-125 DEG C, cooling is concentrated into after can just stirring thereto It is added 50%v/v (600mL), is slowly added dropwise thereto into 50%w/v sodium hydrate aqueous solution, by adding in reaction process The mode of 50%w/v sodium hydrate aqueous solution keeps the pH of reaction solution between 11~12, and reaction temperature is maintained at 40-45 DEG C, Reaction is finished, and concentrated hydrochloric acid is added into reaction solution and adjusts pH3-4, and stirring filters after 3.0 hours, and filter cake is washed with water to neutrality, dries It is dry, obtain white solid 187g, yield 88.2%, HPLC98.14%.
Go step obtained solid (150g, 0.2mol) be added there-necked flask in, thereto be added sodium hydroxide (14.4g, 0.36mol), purified water (300mL), epoxychloropropane (27.9g, 0.30mol) finish 30-35 DEG C of reaction 72.0 hours, instead It should finish, adjust pH3-4, Iodixanol HPLC purity 72.5%, Iohexol HPLC11.3% with concentrated hydrochloric acid.
To sum up, method of the invention is easy to operate, and (III) three obtained formula (I), formula (II) or formula intermediate can be made For the raw material for synthesizing diodone, not by-product truly;Importantly, general sieve of synthesis iodine that can be convenient Amine does not have the generation of two acylated by-products, and compared with original grinds the production technology of medicine, process route is entirely different, high income, cost It is low, a kind of very effective, completely new approach is provided for industrialized production Iopromide, is had a extensive future.
Publication numberPriority datePublication dateAssigneeTitle
WO1998008804A1 *1996-08-291998-03-05Nycomed Imaging AsProcess for iohexol manufacture
US5847212A *1997-04-211998-12-08Abbott LaboratoriesProcess for the preparation of iohexol
WO1999026916A1 *1997-11-261999-06-03Nycomed Imaging AsN-alkylation of 5-amino-2,4,6-triiodo-isophthalamides
ITMI20010773A1 *2001-04-112002-10-11Chemi SpaProcess for the production of high purity iohexole
HAAVALDSEN J ET AL: “X-RAY CONTRAST AGENTS. I. SYNTHESIS OF SOME DERIVATIVES OF 5-AMINO-2, 4, 6-TRIIODOISOPHTHLAMIDE”, ACTA PHARMACEUTICA SUECICA, XX, XX, vol. 20, no. 3, 1983, pages 219 – 232, XP002052827, ISSN: 0001-6675 *
Publication numberPriority datePublication dateAssigneeTitle
WO2007013816A1 *2005-07-292007-02-01Ge Healthcare AsContinuous crystallisation process of iodinated phenyl derivatives
WO2007060380A1 *2005-11-242007-05-31Hovione Inter LtdProcess for the manufacture of iohexol
JP2009502910A *2005-07-292009-01-29ジーイー・ヘルスケア・アクスイェ・セルスカプMethod for continuous crystallization of iodinated phenyl derivatives
CN101195587B *2006-12-192010-07-21浙江尖峰海洲制药有限公司Production method for lodixanol hydrolysate
US8766002B22009-11-262014-07-01Imax Diagnostic Imaging Holding LimitedPreparation and purification of iodixanol
NO342021B1 *2005-07-292018-03-12Ge Healthcare AsContinuous crystallization process
WO2011041275A1 *2009-09-302011-04-07Mallinckrodt Inc.Alkylation of triiodo-substituted arylamides in an aqueous mixed solvent system
ES2680019T3 *2010-12-212018-09-03Ge Healthcare AsDesalination of a composition comprising a contrast agent
US20140065076A1 *2012-08-302014-03-06Otsuka Pharmaceutical Co. Ltd.Container with concentrated substance and method of using the same
* Cited by examiner, † Cited by third party, ‡ Family to family citation
PublicationPublication DateTitle
JP5536087B22014-07-02Method for producing iodinated contrast agent
US5948940A1999-09-07Process for iohexol manufacture
US7541494B22009-06-02Process for the manufacture of iohexol
EP2277855B12011-11-09Crystallization of iodixanol using milling
CA2707173C2011-08-02Crystallization of iodixanol in isopropanol and methanol
CA2710577C2012-09-18Crystallization of iodixanol using milling
References
- ^ Jump up to:a b c d e World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 317–8. hdl:10665/44053. ISBN 9789241547659.
- ^ Jump up to:a b Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 171. ISBN 9781284057560.
- ^ ACR Manual on Contrast Media v10.3. 2017 (PDF). American College of Radiology. 2017. p. 6. ISBN 9781559030120. Archived (PDF) from the original on 1 January 2018. Retrieved 1 January 2018.
- ^ Briggs, Gerald G.; Freeman, Roger K.; Yaffe, Sumner J. (2011). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. p. 761. ISBN 9781608317080. Archived from the original on 1 January 2017.
- ^ Sutton, David; Young, Jeremy W. R. (2012). A Short Textbook of Clinical Imaging. Springer Science & Business Media. p. 235. ISBN 9781447117551. Archived from the original on 1 January 2017.
- ^ Broe, Marc E. de; Porter, George A.; Bennett, William M.; Verpooten, G. A. (2013). Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. Springer Science & Business Media. p. 325. ISBN 9789401590884. Archived from the original on 1 January 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ GE Healthcare (May 2006). “Omnipaque (Iohexol) injection. Product label”. DailyMed. U.S. National Library of Medicine. Retrieved 28 March 2007.
- ^ Amersham Health (April 2006). “Hypaque (Diatrizoate Meglumine and Diatrizoate Sodium) injection, solution. Product label”. DailyMed. U.S. National Library of Medicine. Archived from the original on 23 May 2011. Retrieved 29 March 2007.
- ^ “Omnipaque” (PDF). Ireland: Health Products Regulatory Authority. January 2018. Retrieved 31 July 2020.
- ^ “HistoDenz (D2158)” Archived 2015-11-20 at the Wayback Machine, product information sheet, Sigma-Aldrich. Accessed on line 19 November 2015.
- ^ “Nycodenz®: A universal density gradient medium” Archived 2015-02-26 at the Wayback Machine, Axis-Shield Density Gradient Media. Accessed 19 November 2015.
- ^ Haberfeld H, ed. (2020). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Omnipaque 350 mg J/ml Infusionsflasche.
External links
Iohexol
 |
| Trade names |
Omnipaque, Hexopaque, Oraltag, others |
| Other names |
5-[N-(2,3-Dihydroxypropyl)acetamido]-2,4,6-triiodo-N,N’-bis(2,3-dihydroxypropyl)isophthalamide |
| AHFS/Drugs.com |
Micromedex Detailed Consumer Information |
| License data |
|
Routes of
administration |
intrathecal, intravascular, by mouth, intracavital, rectal |
| ATC code |
|
| Legal status |
- US: ℞-only
- In general: ℞ (Prescription only)
|
| Protein binding |
Low |
| Metabolism |
Nil |
| Elimination half-life |
Variable |
| Excretion |
Kidney, unchanged |
|
|
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard |
100.060.130  |
| Formula |
C19H26I3N3O9 |
| Molar mass |
821.142 g·mol−1 |
| 3D model (JSmol) |
|
| Melting point |
174 to 180 °C (345 to 356 °F) |
|
|
|
|
  (what is this?) (verify) (what is this?) (verify) |
////////////IOHEXOL, Win-39424, Compd 545, Omnipaque, Oraltag, GE Healthcare, X RAY CONTRAST AGENTS, WIN 39424
CC(=O)N(CC(O)CO)C1=C(I)C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C1I