
Isotretinoin
Isotretinoin, also known as 13-cis-retinoic acid and sold under the brand name Accutane among others, is a medication primarily used to treat severe acne. It is also used to prevent certain skin cancers (squamous-cell carcinoma), and in the treatment of other cancers. It is used to treat harlequin-type ichthyosis, a usually lethal skin disease, and lamellar ichthyosis. It is a retinoid, meaning it is related to vitamin A, and is found in small quantities naturally in the body. Its isomer, tretinoin, is also an acne drug.
The most common adverse effects are a transient worsening of acne (lasting 1–4 months), dry lips (cheilitis), dry and fragile skin, and an increased susceptibility to sunburn. Uncommon and rare side effects include muscle aches and pains (myalgias), and headaches. Isotretinoin is known to cause birth defects due to in-utero exposure because of the molecule’s close resemblance to retinoic acid, a natural vitamin A derivative which controls normal embryonic development. It is also associated with psychiatric side effects, most commonly depression but also, more rarely, psychosis and unusual behaviours. Other rare side effects include hyperostosis, and premature epiphyseal closure, have been reported to be persistent.
In the United States, a special procedure is required to obtain the pharmaceutical. In most other countries, a consent form is required which explains these risks. In other countries, such as Israel, it is prescribed like any other medicine from a dermatologist (after proper blood tests).
Women taking isotretinoin must not get pregnant during and for one month after the discontinuation of isotretinoin therapy. Sexual abstinence or effective contraception is mandatory during this period. Barrier methods by themselves (e.g., condoms) are not considered adequate due to the unacceptable failure rates of approximately 3%. Women who become pregnant while taking isotretinoin therapy are generally counseled to have an abortion.
It was patented in 1969 and approved for medical use in 1982.[2] It sold well, but in 2009, Roche decided to discontinue manufacturing due to diminishing market share due to the availability of the many generic versions and the settling of multiple lawsuits over side effects. It continues to be manufactured as of 2019 by Absorica, Amnesteem, Claravis, Myorisan, Sotret, and Zenatane.[3]
Medical uses
Isotretinoin is used primarily for severe cystic acne and acne that has not responded to other treatments.[4][5][6][7] Many dermatologists also support its use for treatment of lesser degrees of acne that prove resistant to other treatments, or that produce physical or psychological scarring.[8] Isotretinoin is not indicated for treatment of prepubertal acne and is not recommended in children less than 12 years of age.[9]
It is also somewhat effective for hidradenitis suppurativa and some cases of severe rosacea.[10] It can also be used to help treat harlequin ichthyosis, lamellar ichthyosis and is used in xeroderma pigmentosum cases to relieve keratoses. Isotretinoin has been used to treat the extremely rare condition fibrodysplasia ossificans progressiva. It is also used for treatment of neuroblastoma, a form of nerve cancer.
Isotretinoin therapy has furthermore proven effective against genital warts in experimental use, but is rarely used for this indication as there are more effective treatments. Isotretinoin may represent an efficacious and safe alternative systemic form of therapy for recalcitrant condylomata acuminata (RCA) of the cervix. In most countries this therapy is currently unapproved and only used if other therapies failed.[11][12]
Prescribing restrictions
Isotretinoin is a teratogen; there is about a 20–35% risk for congenital defects in infants exposed to the drug in utero, and about 30–60% of children exposed to isotretinoin prenatally have been reported to show neurocognitive impairment.[13] Because of this, there are strict controls on prescribing isotretinoin to women who may become pregnant and women who become pregnant while taking isotretinoin are strongly advised to terminate their pregnancies.[13]
In most countries, isotretinoin can only be prescribed by dermatologists or specialist physicians; some countries also allow limited prescription by general practitioners and family doctors. In the United Kingdom[14] and Australia,[15][16] isotretinoin may be prescribed only by or under the supervision of a consultant dermatologist. Because severe cystic acne has the potential to cause permanent scarring over a short period, restrictions on its more immediate availability have proved contentious.[17] In New Zealand, isotretinoin can be prescribed by any doctor but subsidised only when prescribed by a vocationally-registered general practitioner, dermatologist or nurse practitioner.[18]
In the United States, since March 2006 the dispensing of isotretinoin is run through a website called iPLEDGE. The FDA required the companies marketing the drug in the US, which at the time that iPLEDGE was launched were Roche, Mylan, Barr, and Ranbaxy, to put this website in place as a risk evaluation and mitigation strategy. These companies formed a group called the Isotretinoin Products Manufacturing Group, and it hired Covance to run the website.[19][20] Prescribers, pharmacists, and all people to whom the drug is prescribed need to register on the site and log information into it. Women with child-bearing potential must commit to using two forms of effective contraception simultaneously for the duration of isotretinoin therapy and for a month immediately preceding and a month immediately following therapy. Additionally they must have two negative pregnancy tests 30 days apart and have negative pregnancy tests before each prescription is written.[21][22]
History[edit]
The compound 13-cis retinoic acid was first studied in the 1960s at Roche Laboratories in Switzerland by Werner Bollag as a treatment for skin cancer. Experiments completed in 1971 showed that the compound was likely to be ineffective for cancer and, surprisingly, that it could be useful to treat acne. However, they also showed that the compound was likely to cause birth defects, so in light of the events around thalidomide, Roche abandoned the product. In 1975, Gary Peck and Frank Yoder independently rediscovered the drug’s use as a treatment of cystic acne while studying it as a treatment for lamellar ichthyosis, and published that work. Roche resumed work on the drug. In clinical trials, subjects were carefully screened to avoid including women who were or might become pregnant. Roche’s New Drug Application for isotretinoin for the treatment of acne included data showing that the drug caused birth defects in rabbits. The FDA approved the application in 1982.
Scientists involved in the clinical trials published articles warning of birth defects at the same time the drug was launched in the US, but nonetheless isotretinoin was taken up quickly and widely, both among dermatologists and general practitioners. Cases of birth defects showed up in the first year, leading the FDA to begin publishing case reports and to Roche sending warning letters to doctors and placing warning stickers on drug bottles, and including stronger warnings on the label. Lawsuits against Roche started to be filed. In 1983 the FDA’s advisory committee was convened and recommended stronger measures, which the FDA took and were that time unprecedented: warning blood banks not to accept blood from people taking the drug, and adding a warning to the label advising women to start taking contraceptives a month before starting the drug. However use of the drug continued to grow, as did the number of babies born with birth defects. In 1985 the label was updated to include a boxed warning. In early 1988 the FDA called for another advisory committee, and FDA employees prepared an internal memo estimating that around 1,000 babies had been born with birth defects due to isotretinoin, that up to around 1,000 miscarriages had been caused, and that between 5,000 and 7,000 women had had abortions due to isotretinoin. The memo was leaked to the New York Times[77] a few days before the meeting, leading to a storm of media attention. In the committee meeting, dermatologists and Roche each argued to keep the drug on the market but to increase education efforts; pediatricians and the CDC argued to withdraw the drug from the market. The committee recommended to restrict physicians who could prescribe the drug and to require a second opinion before it could be prescribed. The FDA, believing it did not have authority under the law to restrict who had the right to prescribe the drug, kept the drug on the market but took further unprecedented measures: it required to Roche to make warnings yet more visible and graphic, provide doctors with informed consent forms to be used when prescribing the drug, and to conduct follow up studies to test whether the measures were reducing exposure of pregnant women to the drug. Roche implemented those measures, and offered to pay for contraception counseling and pregnancy testing for women prescribed the drug; the program was called the “Pregnancy Prevention Program”.
A CDC report published in 2000[78] showed problems with the Pregnancy Prevention Program and showed that the increase in prescriptions was from off-label use, and prompted Roche to revamp its program, renaming it the “Targeted Pregnancy Prevention Program” and adding label changes like requirements for two pregnancy tests, two kinds of contraception, and for doctors to provide pharmacists with prescriptions directly; providing additional educational materials, and providing free pregnancy tests. The FDA had another advisory meeting in late 2000 that again debated how to prevent pregnant women from being exposed to the drug; dermatologists testified about the remarkable efficacy of the drug, the psychological impact of acne, and demanded autonomy to prescribe the drug; others argued that the drug be withdrawn or much stricter measures be taken. In 2001 the FDA announced a new regulatory scheme called SMART (the System to Manage Accutane Related Teratogenicity) that required Roche to provide defined training materials to doctors, and for doctors to sign and return a letter to Roche acknowledging that they had reviewed the training materials, for Roche to then send stickers to doctors, which doctors would have to place on prescriptions they give people after they have confirmed a negative pregnancy test; prescriptions could only be written for 30 days and could not be renewed, thus requiring a new pregnancy test for each prescription.[citation needed]
In February 2002, Roche’s patents for isotretinoin expired, and there are now many other companies selling cheaper generic versions of the drug. On June 29, 2009, Roche Pharmaceuticals, the original creator and distributor of isotretinoin, officially discontinued both the manufacture and distribution of their Accutane brand in the United States due to what the company described as business reasons related to low market share (below 5%), coupled with the high cost of defending personal-injury lawsuits brought by some people who took the drug.[79] Generic isotretinoin will remain available in the United States through various manufacturers. Roche USA continues to defend Accutane and claims to have treated over 13 million people since its introduction in 1982. F. Hoffmann-La Roche Ltd. apparently will continue to manufacture and distribute Roaccutane outside of the United States.[80]
Among others, actor James Marshall sued Roche over allegedly Accutane-related disease that resulted in removal of his colon.[81] The jury, however, decided that James Marshall had a pre-existing bowel disease.[82]
Several trials over inflammatory bowel disease claims have been held in the United States thus far, with many of them resulting in multimillion-dollar judgments against the makers of isotretinoin.[83]
Society and culture
Brands
As of 2017 isotretinoin was marketed under many brand names worldwide: A-Cnotren, Absorica, Accuran, Accutane, Accutin, Acne Free, Acnecutan, Acnegen, Acnemin, Acneone, Acneral, Acnestar, Acnetane, Acnetin A, Acnetrait, Acnetrex, Acnogen, Acnotin, Acnotren, Acretin, Actaven, Acugen, Acutret, Acutrex, Ai Si Jie, Aisoskin, Aknal, Aknefug Iso, Aknenormin, Aknesil, Aknetrent, Amnesteem, Atlacne, Atretin, Axotret, Casius, Ciscutan, Claravis, Contracné, Curacne, Curacné, Curakne, Curatane, Cuticilin, Decutan, Dercutane, Effederm, Epuris, Eudyna, Farmacne, Flexresan, Flitrion, I-Ret, Inerta, Inflader, Inotrin, Isac, Isdiben, Isoacne, Isobest, Isocural, Isoderm, Isoface, IsoGalen, Isogeril, Isolve, Isoprotil, Isoriac, Isosupra, Isosupra Lidose, Isotane, Isotina, Isotinon, Isotren, Isotret, Isotretinoin, Isotretinoina, Isotretinoína, Isotretinoine, Isotretinoïne, Isotrétinoïne, Isotretinoinum, Isotrex, Isotrin, Isotroin, Izotek, Izotziaja, Lisacne, Locatret, Mayesta, Myorisan, Neotrex, Netlook, Nimegen, Noitron, Noroseptan, Novacne, Oralne, Oraret, Oratane, Piplex, Policano, Procuta, Reducar, Retin A, Roaccutan, Roaccutane, Roacnetan, Roacta, Roacutan, Rocne, Rocta, Sotret, Stiefotrex, Tai Er Si, Teweisi, Tretin, Tretinac, Tretinex, Tretiva, Tufacne, Zenatane, Zerocutan, Zonatian ME, and Zoretanin.[1]
As of 2017 it was marketed as a topical combination drug with erythromycin under the brand names Isotrex Eritromicina, Isotrexin, and Munderm.[1]
Research
While excessive bone growth has been raised a possible side effect, a 2006 review found little evidence for this.[84]
syn

C. D. Robeson et al., J. Am. Chem. Soc. 77, 4111 (1955). Stereoselective process: R. Lucci, EP 111325; idem, US 4556518 (1984, 1985 both to Hoffmann-La Roche). doi:10.1021/jo00349a001.
syn
J Chem Soc 1968,(16),1982-83
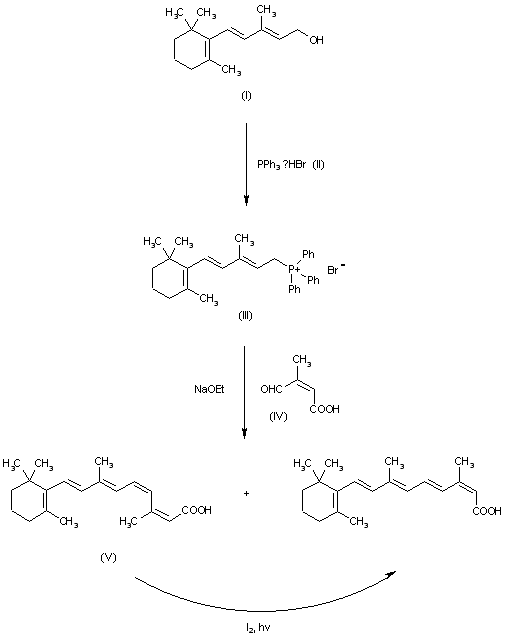
The reaction of vinyl-beta-ionol (I) with triphenylphosphonium bromide (II) in ethanol gives the corresponding phosphonium salt (III), which is condensed through a Wittig reaction with cis-beta-formylcrotonic acid (IV) by means of sodium ethoxide in ethanol to afford a mixture of cis-2-cis-4-vitamin A acid (V) and the desired product. Finally, compound (V) is isomerized bv irradiation with diffuse light in ether in the presence of iodine.
syn
Tetrahedron 2000,56(37),7211
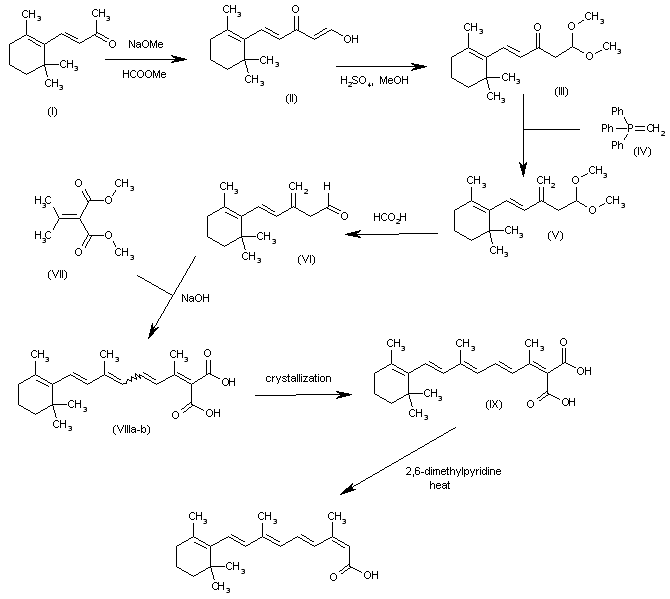
The formylation of the beta-ionone (I) with methyl formate and NaOMe gives the enol (II), which by reaction with methanol and H2SO4 yields the dimethylacetal (III). The reaction of (III) with methylenetriphenylphosphorane (IV) affords the methylene compound (V), which is treated with formic acid to provide the aldehyde (VI). The condensation of (VI) with isopropylidenemalonic acid dimethyl ester (VII) by means of NaOH gives the polyenic malonic acid (VIII) as a mixture of isomers that is separated by crystallization in ethyl ether to yield the desired all-trans-isomer (IX). Finally, this malonic acid is selectively monodecarboxylated by means of refluxing 2,6-dimethylpyridine to afford the target (E,E,E,Z)-isomer.
References
- ^ Jump up to:a b c “Isotretinoin international brands”. Drugs.com. Retrieved 1 June 2017.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 476. ISBN 978-3-527-60749-5.
- ^ “Isotretinoin (Oral Route) Description and Brand Names – Mayo Clinic”.
- ^ Merritt B, Burkhart CN, Morrell DS (June 2009). “Use of isotretinoin for acne vulgaris”. Pediatric Annals. 38 (6): 311–20. doi:10.3928/00904481-20090512-01. PMID 19588674.
- ^ Jump up to:a b Layton A (May 2009). “The use of isotretinoin in acne”. Dermato-Endocrinology. 1(3): 162–9. doi:10.4161/derm.1.3.9364. PMC 2835909. PMID 20436884.
- ^ Jump up to:a b c “Roaccutane 20mg Soft Capsules – Summary of Product Characteristics”. UK Electronic Medicines Compendium. 1 July 2015.
- ^ Jump up to:a b c US Label (PDF) (Report). FDA. 22 October 2010 [January 2010]. Retrieved 1 June2017. See FDA Index page for NDA 018662 for updates
- ^ Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, Thiboutot DM, Van Voorhees AS, Beutner KA, Sieck CK, Bhushan R (April 2007). “Guidelines of care for acne vulgaris management”. Journal of the American Academy of Dermatology. 56 (4): 651–63. doi:10.1016/j.jaad.2006.08.048. PMID 17276540.
- ^ Jump up to:a b c d “Isotretinoin (oral formulations): CMDH scientific conclusions – Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s)”(PDF). European Medicines Agency. August 2017. Retrieved 17 May 2019.
- ^ Jump up to:a b Klasco RK, editor. Drugdex system, vol. 128. Greenwood Village (CO): Thomson Micromedex; 2006.[page needed]
- ^ Georgala S, Katoulis AC, Georgala C, Bozi E, Mortakis A (June 2004). “Oral isotretinoin in the treatment of recalcitrant condylomata acuminata of the cervix: a randomised placebo controlled trial”. Sexually Transmitted Infections. 80 (3): 216–8. doi:10.1136/sti.2003.006841. PMC 1744851. PMID 15170007.
- ^ Sehgal VN, Srivastava G, Sardana K (June 2006). “Isotretinoin–unapproved indications/uses and dosage: a physician’s reference”. International Journal of Dermatology. 45 (6): 772–7. doi:10.1111/j.1365-4632.2006.02830.x. PMID 16796650.
- ^ Jump up to:a b Choi JS, Koren G, Nulman I (March 2013). “Pregnancy and isotretinoin therapy”. Canadian Medical Association Journal. 185 (5): 411–3. doi:10.1503/cmaj.120729. PMC 3602257. PMID 23296582.
- ^ Joint Formulary Committee. British National Formulary (47th ed.). London: British Medical Association and Royal Pharmaceutical Society of Great Britain. ISBN 978-0-85369-584-4.[page needed]
- ^ “Fresh call for GPs to prescribe Roaccutane”. AustralianDoctor. 19 June 2012.
- ^ Specifically, doctors who are fellows of the Australasian College of Dermatologists (FACD); cf. Pharmaceutical Services Branch, Guide to poisons and therapeutic goods legislation for medical practitioners and dentists, Sydney: NSW Department of Health; 2006.[page needed]
- ^ James M (June 1996). “Isotretinoin for severe acne”. Lancet. 347 (9017): 1749–50. doi:10.1016/S0140-6736(96)90814-4. PMID 8656912. S2CID 28756302.
- ^ “Acne, Isotretinoin, and Depression”. MEDSAFE (New Zealand Ministry of Health). June 2013 [June 2005]. Retrieved 7 February 2014.
- ^ Thiboutot, D. M.; Cockerell, C. J. (1 August 2006). “iPLEDGE: A Report from the Front Lines of Dermatologic Practice”. AMA Journal of Ethics. 8 (8): 524–528. doi:10.1001/virtualmentor.2006.8.8.pfor1-0608. ISSN 1937-7010. PMID 23234692.
- ^ Darves, Bonnie (March 9, 2006). “Dermatologists Frustrated With Problematic iPledge Program”. Medscape.
- ^ “iPledge (About iPledge)”.
- ^ “Isotretinoin (marketed as Accutane) Capsule Information”. U.S. Food and Drug Administration (FDA). 2018-11-03.
- ^ Jump up to:a b c “Isotretinoin 20mg capsules – – (eMC)”. www.medicines.org.uk. Retrieved 2017-12-27.
- ^ “Isotretinoin 20mg capsules – – (eMC)”. www.medicines.org.uk. Retrieved 2018-01-10.
- ^ David M, Hodak E, Lowe NJ (1988). “Adverse effects of retinoids”. Medical Toxicology and Adverse Drug Experience. 3 (4): 273–88. doi:10.1007/bf03259940. PMID 3054426. S2CID 12432684.
- ^ DiGiovanna JJ (November 2001). “Isotretinoin effects on bone”. Journal of the American Academy of Dermatology. 45 (5): S176-82. doi:10.1067/mjd.2001.113721. PMID 11606950.
- ^ Ellis CN, Madison KC, Pennes DR, Martel W, Voorhees JJ (1984). “Isotretinoin therapy is associated with early skeletal radiographic changes”. Journal of the American Academy of Dermatology. 10 (6): 1024–9. doi:10.1016/S0190-9622(84)80329-1. PMID 6588057.
- ^ “Isotretinoin risks in acne treatment: Page 3 of 4”. October 2014.
- ^ Jump up to:a b Moy A, McNamara NA, Lin MC (September 2015). “Effects of Isotretinoin on Meibomian Glands”. Optometry and Vision Science. 92 (9): 925–30. doi:10.1097/OPX.0000000000000656. PMID 26154692. S2CID 205905994.
- ^ Jump up to:a b Lambert RW, Smith RE (March 1989). “Effects of 13-cis-retinoic acid on the hamster meibomian gland”. The Journal of Investigative Dermatology. 92 (3): 321–5. doi:10.1111/1523-1747.ep12277122. PMID 2918239.
- ^ Fraunfelder FT, Fraunfelder FW, Edwards R (September 2001). “Ocular side effects possibly associated with isotretinoin usage”. American Journal of Ophthalmology. 132 (3): 299–305. doi:10.1016/S0002-9394(01)01024-8. PMID 11530040.
- ^ Jump up to:a b c d e f g h Brelsford M, Beute TC (September 2008). “Preventing and managing the side effects of isotretinoin”. Seminars in Cutaneous Medicine and Surgery. 27 (3): 197–206. doi:10.1016/j.sder.2008.07.002. PMID 18786498.
- ^ Scheinfeld N, Bangalore S (May 2006). “Facial edema induced by isotretinoin use: a case and a review of the side effects of isotretinoin”. Journal of Drugs in Dermatology. 5 (5): 467–8. PMID 16703787.
- ^ Jump up to:a b “Updated measures for pregnancy prevention during retinoid use”. European Medicines Agency. 21 June 2018.
- ^ Roche Products Pty Ltd. Roaccutane (Australian Approved Product Information). Dee Why (NSW): Roche; 2005.[page needed]
- ^ Leyden JJ, Del Rosso JQ, Baum EW (February 2014). “The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions”. The Journal of Clinical and Aesthetic Dermatology. 7 (2 Suppl): S3–S21. PMC 3970835. PMID 24688620.
- ^ BNF, edition 57[page needed]
- ^ Jump up to:a b c d e f g h i j k l m Bremner JD, Shearer KD, McCaffery PJ (January 2012). “Retinoic acid and affective disorders: the evidence for an association”. The Journal of Clinical Psychiatry (Systematic Review). 73 (1): 37–50. doi:10.4088/JCP.10r05993. PMC 3276716. PMID 21903028.
- ^ Jump up to:a b c Kontaxakis VP, Skourides D, Ferentinos P, Havaki-Kontaxaki BJ, Papadimitriou GN (January 2009). “Isotretinoin and psychopathology: a review”. Annals of General Psychiatry. 8: 2. doi:10.1186/1744-859X-8-2. PMC 2637283. PMID 19154613.
- ^ Jump up to:a b c d Borovaya A, Olisova O, Ruzicka T, Sárdy M (September 2013). “Does isotretinoin therapy of acne cure or cause depression?”. International Journal of Dermatology. 52 (9): 1040–52. doi:10.1111/ijd.12169. PMID 23962262.
- ^ Jump up to:a b “Interactive Drug Analysis Profile – Isotretinoin”. mhra.gov.uk. Medicines & Healthcare Products Regulatory Agency. 31 March 2017.
- ^ Jump up to:a b Goodfield MJ, Cox NH, Bowser A, McMillan JC, Millard LG, Simpson NB, Ormerod AD (June 2010). “Advice on the safe introduction and continued use of isotretinoin in acne in the U.K. 2010”. The British Journal of Dermatology. 162 (6): 1172–9. doi:10.1111/j.1365-2133.2010.09836.x. PMID 21250961.
- ^ Jump up to:a b Ludot M, Mouchabac S, Ferreri F (June 2015). “Inter-relationships between isotretinoin treatment and psychiatric disorders: Depression, bipolar disorder, anxiety, psychosis and suicide risks”. World Journal of Psychiatry. 5 (2): 222–7. doi:10.5498/wjp.v5.i2.222. PMC 4473493. PMID 26110123.
- ^ Wysowski DK, Pitts M, Beitz J (October 2001). “An analysis of reports of depression and suicide in patients treated with isotretinoin”. Journal of the American Academy of Dermatology. 45 (4): 515–9. doi:10.1067/mjd.2001.117730. PMID 11568740.
- ^ Jump up to:a b Rowe C, Spelman L, Oziemski M, Ryan A, Manoharan S, Wilson P, Daubney M, Scott J (May 2014). “Isotretinoin and mental health in adolescents: Australian consensus”. The Australasian Journal of Dermatology (Review). 55 (2): 162–7. doi:10.1111/ajd.12117. PMID 24283385. S2CID 29178483.
- ^ Palha JA, Goodman AB (June 2006). “Thyroid hormones and retinoids: a possible link between genes and environment in schizophrenia” (PDF). Brain Research Reviews. 51(1): 61–71. doi:10.1016/j.brainresrev.2005.10.001. hdl:1822/3943. PMID 16325258. S2CID 30773986.
- ^ Jump up to:a b c d Goodman AB (March 1994). “Retinoid dysregulation as a cause of schizophrenia”. The American Journal of Psychiatry. 151 (3): 452–3. doi:10.1176/ajp.151.3.452b. PMID 8109664.
- ^ Goodman AB (May 1996). “Congenital anomalies in relatives of schizophrenic probands may indicate a retinoid pathology”. Schizophrenia Research. 19 (2–3): 163–70. doi:10.1016/0920-9964(96)88523-9. PMID 8789914. S2CID 12089905.
- ^ Goodman AB (July 2005). “Microarray results suggest altered transport and lowered synthesis of retinoic acid in schizophrenia”. Molecular Psychiatry. 10 (7): 620–1. doi:10.1038/sj.mp.4001668. PMID 15838536.
- ^ Samad TA, Krezel W, Chambon P, Borrelli E (December 1997). “Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor-retinoid X receptor family”. Proceedings of the National Academy of Sciences of the United States of America. 94 (26): 14349–54. Bibcode:1997PNAS…9414349S. doi:10.1073/pnas.94.26.14349. PMC 24972. PMID 9405615.
- ^ Crockett SD, Porter CQ, Martin CF, Sandler RS, Kappelman MD (September 2010). “Isotretinoin use and the risk of inflammatory bowel disease: a case-control study”. The American Journal of Gastroenterology. 105 (9): 1986–93. doi:10.1038/ajg.2010.124. PMC 3073620. PMID 20354506.
- ^ Lowenstein EB, Lowenstein EJ (2011). “Isotretinoin systemic therapy and the shadow cast upon dermatology’s downtrodden hero”. Clinics in Dermatology. 29 (6): 652–61. doi:10.1016/j.clindermatol.2011.08.026. PMID 22014987.
- ^ “Drug Safety Update – Latest advice for medicines users – October 2017” (PDF). Medicines and Healthcare products Regulatory Agency. 3 October 2017. Retrieved 17 May2019.
- ^ “Pharmacovigilance Risk Assessment Committee (PRAC) – Minutes for the meeting on 3–6 July 2017” (PDF). European Medicines Agency. 1 September 2017. p. 44. Retrieved 17 May 2019.
- ^ Kremer I, Gaton DD, David M, Gaton E, Shapiro A (1994). “Toxic effects of systemic retinoids on meibomian glands”. Ophthalmic Research. 26 (2): 124–8. doi:10.1159/000267402. PMID 8196934.
- ^ Griffin JN, Pinali D, Olds K, Lu N, Appleby L, Doan L, Lane MA (November 2010). “13-Cis-retinoic acid decreases hypothalamic cell number in vitro”. Neuroscience Research. 68 (3): 185–90. doi:10.1016/j.neures.2010.08.003. PMID 20708044. S2CID 207152111.
- ^ Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P (April 2004). “13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice”. Proceedings of the National Academy of Sciences of the United States of America. 101 (14): 5111–6. Bibcode:2004PNAS..101.5111C. doi:10.1073/pnas.0306336101. JSTOR 3371827. PMC 387382. PMID 15051884.
- ^ Sakai Y, Crandall JE, Brodsky J, McCaffery P (June 2004). “13-cis Retinoic acid (accutane) suppresses hippocampal cell survival in mice”. Annals of the New York Academy of Sciences. 1021 (1): 436–40. Bibcode:2004NYASA1021..436S. doi:10.1196/annals.1308.059. PMID 15251924.
- ^ Nelson AM, Cong Z, Gilliland KL, Thiboutot DM (September 2011). “TRAIL contributes to the apoptotic effect of 13-cis retinoic acid in human sebaceous gland cells”. The British Journal of Dermatology. 165 (3): 526–33. doi:10.1111/j.1365-2133.2011.10392.x. PMC 3166444. PMID 21564055.
- ^ Nelson AM, Gilliland KL, Cong Z, Thiboutot DM (October 2006). “13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes”. The Journal of Investigative Dermatology. 126 (10): 2178–89. doi:10.1038/sj.jid.5700289. PMID 16575387.
- ^ Wachter K (2009). “Isotretinoin’s Mechanism of Action Explored”. Skin & Allergy News. 40(11): 32. doi:10.1016/S0037-6337(09)70553-4.
- ^ Isotretinoin’s Mechanism of Action Elucidated Archived 2010-04-04 at the Wayback Machine. Medconnect (2009-08-28). Retrieved on 2010-11-13.
- ^ Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM (April 2008). “Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells”. The Journal of Clinical Investigation. 118 (4): 1468–78. doi:10.1172/JCI33869. PMC 2262030. PMID 18317594.
- ^ Jump up to:a b Peck GL, Olsen TG, Yoder FW, Strauss JS, Downing DT, Pandya M, Butkus D, Arnaud-Battandier J (February 1979). “Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid”. The New England Journal of Medicine. 300 (7): 329–33. doi:10.1056/NEJM197902153000701. PMID 153472.
- ^ Shalita A (2001). “The integral role of topical and oral retinoids in the early treatment of acne”. Journal of the European Academy of Dermatology and Venereology. 15: 43–9. doi:10.1046/j.0926-9959.2001.00012.x. PMID 11843233.
- ^ [unreliable medical source?]Farrell LN, Strauss JS, Stranieri AM (December 1980). “The treatment of severe cystic acne with 13-cis-retinoic acid. Evaluation of sebum production and the clinical response in a multiple-dose trial”. Journal of the American Academy of Dermatology. 3 (6): 602–11. doi:10.1016/S0190-9622(80)80074-0. PMID 6451637.
- ^ [unreliable medical source?]Jones H, Blanc D, Cunliffe WJ (November 1980). “13-cis retinoic acid and acne”. Lancet. 2 (8203): 1048–9. doi:10.1016/S0140-6736(80)92273-4. PMID 6107678. S2CID 40877032.
- ^ Pendino F, Flexor M, Delhommeau F, Buet D, Lanotte M, Segal-Bendirdjian E (June 2001). “Retinoids down-regulate telomerase and telomere length in a pathway distinct from leukemia cell differentiation”. Proceedings of the National Academy of Sciences of the United States of America. 98 (12): 6662–7. Bibcode:2001PNAS…98.6662P. doi:10.1073/pnas.111464998. JSTOR 3055868. PMC 34517. PMID 11371621.
- ^ Φαχαντίδης, Παναγιώτης Ε. (2007). Η επίδραση της ισοτρετινοϊνης και των αναστολέων της 5α-αναγωγάσης στις μεταλλοπρωτεάσες του συνδετικού ιστού σε ασθενείς με ακμή[The influence of isotretinoin and 5-a reductase inhibitors in metaloproteases of connective tissue in patients with ance] (in Greek). Aristotle University of Thessaloniki.[unreliable medical source?]
- ^ Toyoda M, Nakamura M, Makino T, Kagoura M, Morohashi M (June 2002). “Sebaceous glands in acne patients express high levels of neutral endopeptidase”. Experimental Dermatology. 11 (3): 241–7. doi:10.1034/j.1600-0625.2002.110307.x. PMID 12102663. S2CID 23468315.
- ^ Wysowski DK, Swartz L (May 2005). “Relationship between headache and depression in users of isotretinoin”. Archives of Dermatology. 141 (5): 640–1. doi:10.1001/archderm.141.5.640. PMID 15897395.
- ^ Magin P, Pond D, Smith W (February 2005). “Isotretinoin, depression and suicide: a review of the evidence”. The British Journal of General Practice. 55 (511): 134–8. PMC 1463189. PMID 15720936.
- ^ Ng CH, Schweitzer I (February 2003). “The association between depression and isotretinoin use in acne”. The Australian and New Zealand Journal of Psychiatry. 37 (1): 78–84. doi:10.1046/j.1440-1614.2003.01111.x. PMID 12534661. S2CID 8475675.
- ^ Jump up to:a b c d e “FDA information, side effects, and uses / Accutane (isotretinoin)”. U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- ^ “FDA information, side effects, and uses / Accutane (isotretinoin) : Table 2 Pharmacokinetic Parameters of Isotretinoin Mean (%CV), N=74“. U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- ^ “FDA information, side effects, and uses / Accutane (isotretinoin) : Drug Interactions“. U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- ^ Gina Kolata for the New York Times. April 22, 1988 Anti-Acne Drug Faulted in Birth
- ^ CDC. January 21, 2000 Accutane®-Exposed Pregnancies — California, 1999 MMWR Weekly 49(02);28-31
- ^ Shari Roan (7 November 2009). “New study may deal final blow to acne drug Accutane”. LA Times.
- ^ “Roche Discontinues and Plans to Delist Accutane in the U.S.” (Press release). Genentech. 2009-06-29. Archived from the original on 2009-11-08. Retrieved 2010-11-12.
- ^ Feeley J (2011-03-11). “Roche Accutane Acne Drug Caused ‘Tragedy’ for Actor, Brian Dennehy Says”. Bloomberg.
- ^ Silverman E (2011-11-04). “It’s Curtains On Actor’s Accutane Lawsuit”. Pharmalot. UBM Canon.
- ^ Voreacos D (May 30, 2007). “Roche Found Liable in First Of 400 Suits Over Accutane”. The Washington Post. Bloomberg News. Retrieved April 30, 2012.
- ^ Halverstam CP, Zeichner J, Lebwohl M (2006). “Lack of significant skeletal changes after long-term, low-dose retinoid therapy: case report and review of the literature”. Journal of Cutaneous Medicine and Surgery. 10 (6): 291–9. doi:10.2310/7750.2006.00065. PMID 17241599. S2CID 36785828.
External links
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | See note at tretinoin |
| Trade names | Accutane, Roaccutane, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681043 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Variable |
| Protein binding | 99.9% |
| Metabolism | Liver |
| Elimination half-life | 10–20 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.022.996 |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
////////////Antiacne, 13-cis-Retinoic acid, 2-cis-vitamin A acid, neovitamin A acid, Isotretinoin














