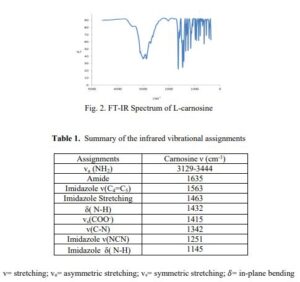
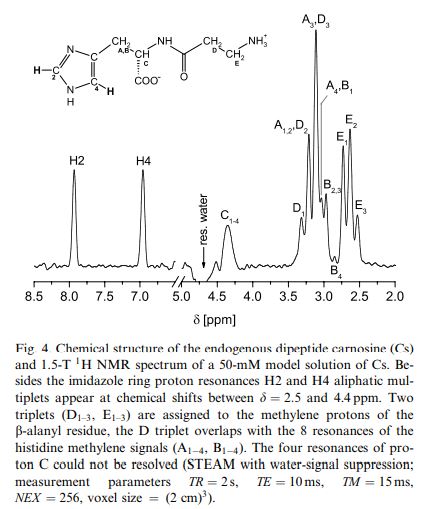
L-CARNOSINE
- Molecular FormulaC9H14N4O3
- Average mass226.232 Da
Carnosine (beta-alanyl-L-histidine) is a dipeptide molecule, made up of the amino acids beta-alanine and histidine. It is highly concentrated in muscle and brain tissues.[citation needed] Carnosine was discovered by Russian chemist Vladimir Gulevich.[2]
Carnosine is naturally produced by the body in the liver[3] from beta-alanine and histidine. Like carnitine, carnosine is composed of the root word carn, meaning “flesh”, alluding to its prevalence in meat.[4] There are no plant-based sources of carnosine,[5] however synthetic supplements do exist.
AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT
SYN
WO2009033754 PAGE: 98 claimed protein
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009033754
SYN
Showa Igakkai Zasshi 1974, V34(3), P271-83
Russian Journal of General Chemistry 2007, V77(9), P1576-1579
Chemische Berichte 1961, V94, P2768-78
Farmaco, Edizione Scientifica 1968, V23(9), P859-69
Paper
Journal of the American Chemical Society 1953, V75, P2388-90
| +21.9 ° |
Conc: 3.0 g/100mL;water ; Wavlenght: 589.3 nm; Temp: 20 °C
Annali di Chimica (Rome, Italy) 1968, V58(11), P1431-4
Z. physiol. Chem. 1914, V87, P1-11
PAPER
Chemistry – A European Journal (2003), 9, (8), 1714-1723.
PAPER
Journal of Magnetic Resonance (2003), 164, (2), 256-269.
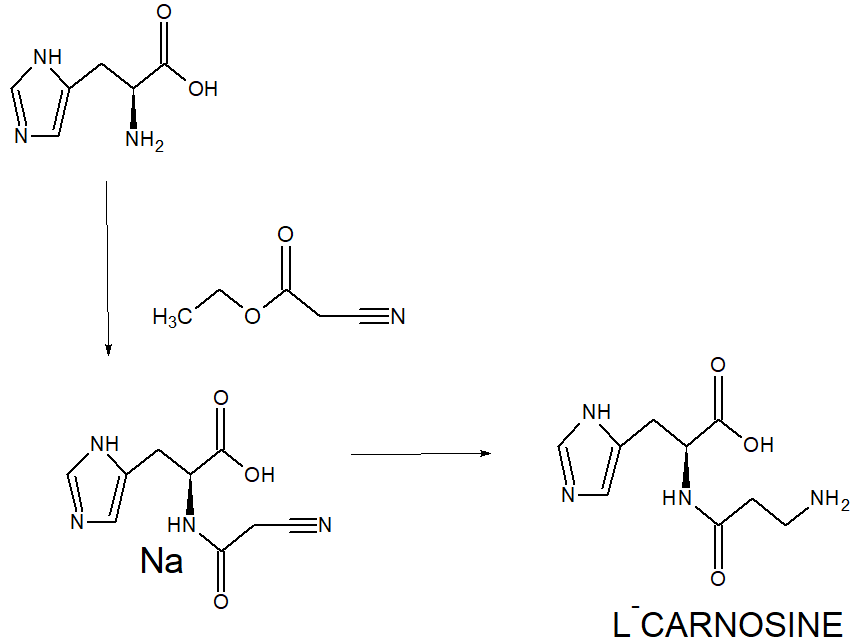
SYN
WO 2001064638
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2001064638
Example 1
(S) -2- (Cyanoacetylamino) -3- (l_ * H-imidazol-4-yl) propionic acid, sodium salt
To a solution of sodium ethoxide obtained by dissolving 5.57 g (0.24 mol) of sodium in 800 ml of ethanol was added 40.0 g (0.26 mol) of L-histidine at room temperature. After 15 minutes, 44.12 g (0.39 mol) of ethyl cyanoacetate were added and the suspension was refluxed for 16 hours. After cooling to room temperature, the mixture was filtered. The yellowish filtrate was concentrated in vacuo, the residue was slurried in ethyl acetate, filtered, washed with ethyl acetate and purified by flash chromatography on silica gel (eluent: gradient ethyl acetate → methanol / ethyl acetate 3: 1).
Yield: 28.42 g (46%)
1HNMR (DMSO- ^ 6, 00 MHz): δ = 8,28 (d, 1H); 7,45 (s, 1H); 6,7 (s, 1H); 5,5 (br. s, 1H); 4,12-4,20 (m, 1H); 3,65 (s, 2H); 2,95-3,05 (m, 1H); 2,8-2,9 (m, 1H).
13C NMR (DMSO- 6, 100 MHz): δ = 174,05; 161,09; 134,25; 131,97; 119,66; 116,43; 54,83; 29,13; 25,20.
Example 2
(• S) -2- (Cyanoacetylamino) -3- (1-δ-imidazol-4-yl) propionic acid, sodium salt
9.80 g of sodium hydride (60% in mineral oil) and 50.6 g
(0.51 mol) were added at room temperature to a suspension of 40.0 g (0.26 mol) of L-histidine in 750 ml of N, N-dimethylformamide Given methyl cyanoacetate. The mixture was heated to 155 ° C. for 2 h in an open flask and the solution thus obtained was analyzed by means of HPLC.
Histidine (8 area%) and (S) -2- (cyanoacetylamino) -3- (1H-imidazol-4-yl) propionic acid sodium salt (38 area%) were identified.
Example 3
(S) -2- (Cyanoacetylamino) -3- (l-ö r -imidazol-4-yl) propionic acid
To a solution of sodium ethoxide obtained by dissolving 4.02 g (0.175 mol) of sodium in 280 ml of ethanol, 28.27 g (0.18 mol) of L-Ηistidine were added at room temperature. The mixture was heated slowly and 30.92 g (0.27 mol) of ethyl cyanoacetate were added dropwise at a temperature of 60.degree. The mixture was heated further and the ethanol was distilled off, the amount of ethanol distilled off being continuously replaced in portions by N, N-dimethylformamide. At the end of the reaction, the temperature of the solution was 130 ° C. The mixture was stirred at this temperature for a further 2 hours. The brown reaction mixture (200 g) was cooled to 50 ° C. and 30 g of concentrated hydrochloric acid were metered in. About 70 g of solvent (Η 2O / N, N-dimethylformamide mixture) distilled off. The viscous suspension was mixed with 200 g of acetone, cooled to -10 ° C. and filtered. For recrystallization, the residue was dissolved in water and the pH was adjusted to 5.0. On cooling (<5 ° C.) a white solid precipitated out, which was filtered off, washed with ethanol and dried at 40 ° C./20 mbar.
Yield: 26.39 g (66%).
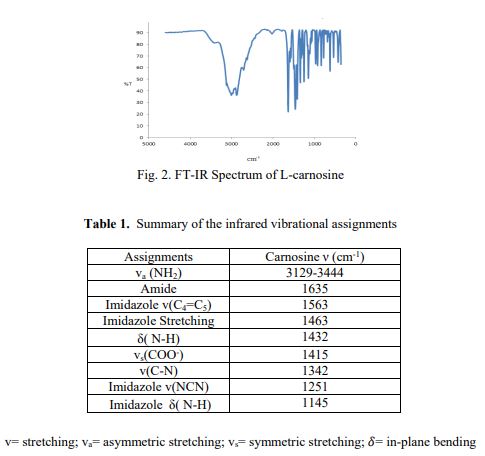
IR (KBr): v = 3421, 3240, 3149, 3059, 2970, 2255, 1653, 1551, 1396, 1107, 1088, 979, 965, 826, 786, 638 cm is “1 .
1HΝMR (DMSO-c 6 , 400 MHz): δ = 11.0 (br., 2H); 8.50 (d, 1H); 7.68 (s, 1H); 6.85 (s, 1H); 4.35-4.48 ( m, 1H); 3.68 (s, 2H); 2.92-3.03 (, 1H); 2.82-2.91 (m, 1H).
13 C NMR (DMSO- 6 , 100 MHz): δ = 172.23; 161.92; 134.55; 132.70; 116.73; 115.87; 52.80; 28.68; 25.06.
LC-MS: mlz = 223 ([M + H]), 205, 177, 156, 110.
The optical purity was determined to be> 99.8% on a sample obtained according to the above procedure. The determination was carried out by hydrolysis of the amide bond (6 N hydrochloric acid, 110 ° C., 24 h), followed by derivatization of the released histidine with trifluoroacetic anhydride and isobutyl chloroformate. A D-histidine content of <0.1% was detected by gas chromatography on a chiral stationary phase.
Example 4
L-Carnosine
To a solution of 1.90 g (7.8 mmol) of (<S) -2- (cyanoacetylamino) -3- (1H-imidazol-4-yl) propionic acid sodium salt (prepared according to Example 1) in 50 ml of ethanol / conc.
Ammonia solution (V: V- 4: 1) were given 0.3 g of rhodium / activated charcoal (5% Rh). The
The mixture was hydrogenated at 110 ° C. and 45 bar for 1 hour. The catalyst was then filtered off and the filtrate was adjusted to pH 8.2 with formic acid. After the solution had been concentrated in vacuo, the residue was suspended in 200 ml of ethanol and heated to 60 ° C. for 30 minutes. The product was filtered off, washed successively with ethanol, ethyl acetate and diethyl ether and finally dried.
Yield: 1.33 g (76%)
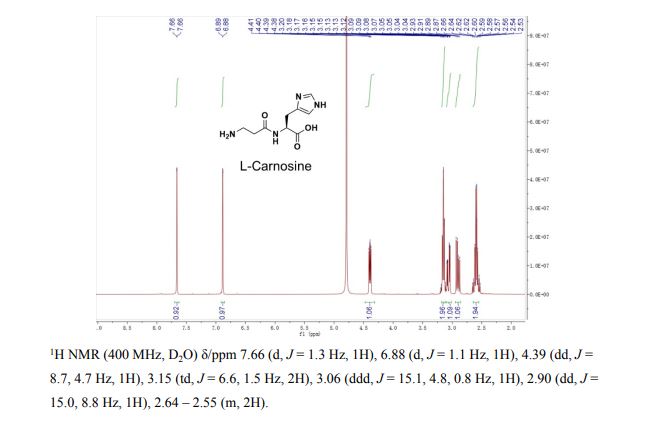
1H NMR (D 2 O, 400 MΗz): δ = 7.70 (s, 1Η); 6.93 (s, 1Η); 4.43-4.50 (m, 1Η); 3.20-3.28 (m, 2Η); 3.11-3.19 (m, 1H); 2.95-3.03 (m, 1H); 2.61-2.71 (m, 2H).
The optical purity was determined by the method described in Example 3 to be 99.5%.
Example 5
(S) -2- (Cyanoacetylamino) -3- (1-O-imidazol-4-yl) propionic acid methyl ester
To a solution of sodium methoxide obtained by dissolving 0.94 g (40.7 mmol; 1.95 equiv.) Of sodium in 100 ml of methanol, 5.0 g (20.4 mmol) were added at room temperature
L-histidine methyl ester dihydrochloride added. After 30 minutes, 3.03 g
(30.6 mmol) of methyl cyanoacetate were added and the mixture was left on for 16 hours
Boiled under reflux. After cooling to room temperature, the mixture was filtered.
The yellowish filtrate was concentrated in vacuo and the residue was purified by means of flash chromatography on silica gel (eluent: gradient ethyl acetate – »ethyl acetate / methanol 3: 1).
Yield: 1.51 g (31%)
1H MR (OMSO-de, 400 MHz): δ = 8.65 (d, 1H); 7.52 (s. 1H); 6.8 (s, 1H); 4.45 ^ 1.55 (m,
1H); 3,69 (s, 2H); 3,62 (s, 3H); 3,3 (br., 1H); 2,82-2,98 (m, 2H).
Example 6
L-Carnosine
1.76 g of Rh / C (0.4 mol% of pure Rh based on the starting material used) in a mixture of 94.2 g of ammonia solution (25% in H 2 O) and 62.8 g of methanol were placed in a 1 liter pressure autoclave . The autoclave was closed, the contents were heated to 90 ° C. and 40 bar hydrogen was injected. A solution of 20.0 g (0.09 mol) (* S) -2- (cyanoacetylamino) -3- (1H-imidazol-4-yl) propionic acid (prepared according to Example 3) was then within one hour Mixture 94.2 g ammonia solution (25% in Η 2O) and 62.8 g of methanol are metered in. After a one hour post-reaction at 90 ° C., the reaction mixture was cooled to room temperature. The pressure in the autoclave was released and the catalyst was filtered off over activated charcoal. An HPLC in-process analysis showed that the clear greenish reaction solution (326.2 g) contained 5.74% (m / m) carnosine, which corresponds to a selectivity of 92% with complete conversion. The reaction mixture was then concentrated to approx. 60 g on a rotary evaporator. As a result of the dropwise addition of 174 g of ethanol, a white solid precipitated out, which was filtered off and dried at 50 ° C./20 mbar.
Ausbeute: 13,0 g (64%)
1H NMR (D2O, 400 MHz): δ = 7,70 (s, 1H); 6,93 (s, 1H); 4,43-4,50 (m, 1H); 3,20-3,28 (m, 2H); 3,11-3,19 (m, 1H); 2,95-3,03 (m, 1H); 2,61-2,71 (m, 2H).
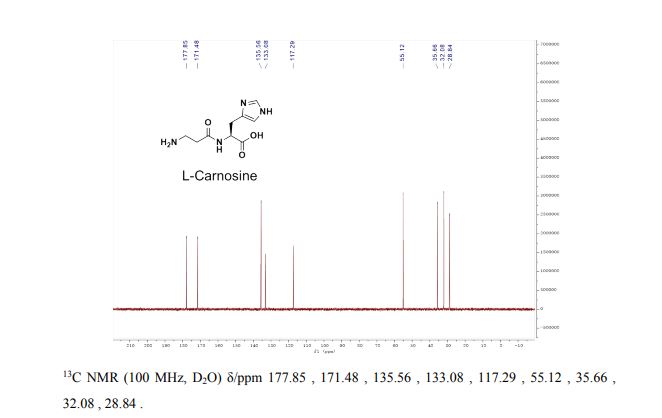
I3C NMR (D20, 100 MHz): δ = 178,58; 172,39; 136,46; 133,90; 118,37; 55,99; 36,65; 33,09; 29,74.
LC-MS: m/z = 227 ([M+H]+), 210, 192, 164, 146, 136, 110.
Example 7
L-Carnosine
In a 1 liter pressure autoclave, a solution of 10.00 g (45.0 mmol) (S) -2- (cyanoacetylamino) -3 was added to 0.88 g of Rh / C (0.4 mol% of pure Rh based on the starting material used) – (1H-imidazol-4-yl) propionic acid (prepared according to Example 3) in a mixture of 157 g conc. NΗ 3/ Methanol (m / m = 3: 2) was added. The autoclave was closed and flushed twice with 40 bar nitrogen and once with hydrogen. The mixture was heated to 90 ° C. and 40 bar hydrogen was injected. After 3 h at 90 ° C., the reaction mixture was cooled to room temperature, the autoclave was depressurized and the catalyst was separated off by filtration. An in-process analysis (HPLC) showed that the reaction solution (147.2 g) contained 6.38% (m / m) carnosine, which corresponds to a selectivity of 92% when the conversion is complete. The reaction mixture was then concentrated to 41.2 g on a rotary evaporator. 124 g of ethanol were added dropwise at room temperature and the flask was placed in a refrigerator overnight. The next day the precipitate was filtered off, washed with ethanol and dried in a drying cabinet at 40 ° C./20 mbar. 7.96 g (78%) of a slightly greenish solid with a content (HPLC) of 98.0% (m / m) were obtained.
Example 8
L-Carnosine
The procedure was as described in Example 7, with the difference that 5% Rh on aluminum oxide was used as the catalyst. Under these conditions, L-carnosine was formed with 83% selectivity.
Example 9
L-Carnosine
4.5 g of Raney cobalt (doped with 0.3% iron) in 195 g of methanol were placed in a 1 liter pressure autoclave. A solution of 30.0 g (0.135 mol) (S) -2- (cyanoacetylamino) -3- (1H-imidazol-4-yl) propionic acid (prepared according to Example 3) in 375 g ammonia solution (25% in Η O) was admitted. The autoclave was closed and flushed twice with 40 bar nitrogen. Then 45 bar of hydrogen were injected and the contents were heated to 100 ° C. within half an hour. After an after-reaction of 3 hours at 100 ° C., the reaction mixture was cooled to room temperature and the pressure in the autoclave was released. An HPLC in-process analysis showed that the reaction solution (590.8 g) contained 4.68% (mim) carnosine, which corresponds to a selectivity of 91% with complete conversion.
Example 10
L-Carnosine
In a 100 ml
pressure autoclave were to a solution of 2.0 g (9.0 mmol) (ιS) -2- (cyanoacetylamino) -3- (lH-imidazol-4-yl) propionic acid (prepared according to Example 3) in a Mixture of 25 g of ammonia solution (25% in Η 2 O) and 13 g of methanol, 1.1 g of Raney nickel (doped with 1.8% molybdenum) were added. The autoclave was closed and placed in an oil bath preheated to 100.degree. After 10 minutes, 50 bar of hydrogen were injected. After 2.5 hours at 100 ° C., the reaction mixture was
cooled to room temperature and the pressure on the autoclave was released. An HPLC in-process analysis showed that the reaction solution (39.4 g) contained 4.54% (m / m) carnosine, which, with a conversion of 99%, corresponds to a selectivity of 89%.
Example 11
L-Carnosine
In a 1 liter pressure autoclave, 4.50 g of Raney cobalt (doped with 0.3% iron) in a mixture of 285 g of conc. Ammonia / methanol (mim = 1.9: 1) submitted. The autoclave was closed and flushed twice with 40 bar nitrogen. Then 45 bar of hydrogen were injected and the mixture was heated to 100.degree. A solution of 30.0 g (0.135 mol) of (S) -2- (cyanoacetylamino) -3- (1H-imidazol-4-yl) propionic acid (prepared according to Example 3) in a mixture of 285 g was then obtained within one hour conc. Ammonia / methanol (m / m = 1.9: 1) metered in. After a one hour post-reaction at 100 ° C., the reaction mixture was cooled to room temperature. The pressure in the autoclave was released and the catalyst was filtered off. A ΗPLC in-process analysis showed that the reddish brown reaction solution (310.5 g) contained 9.57% (m / m) carnosine,
Example 12
(S) -2- (Cyanoacetylamino) -3- (3-methyl-3-ö r -imidazol-4-yl) propionic acid, sodium salt
0.50 g (2.95 mmol) of 3-methyl-L-histidine were added at 40 ° C. to a solution of 0.20 g (2.94 mmol) of sodium ethoxide in 5.60 g of ethanol. The clear solution was heated to 60 ° C. and 0.50 g (4.43 mmol) ethyl cyanoacetate was added dropwise. The mixture was refluxed for 1 hour. Then 10 mg (0.15 mmol) of imidazole were added. The ethanol was then slowly distilled off and the amount of ethanol distilled off was continuously replaced in portions by N, N-dimethylformamide. After a subsequent reaction time of 2 h at 125 ° C., the reaction mixture was carefully concentrated and the residue was purified by means of flash column chromatography on silica gel (eluent: gradient ethyl acetate → ethyl acetate / methanol 2: 1). 0.49 g (64%) of a slightly yellowish solid were obtained.
DC: Rf •= 0,46 (Ethanol/H2O 3:7).
1H NMR (DMSO-öfe, 400 MHz): δ = 7,91 (d, 1H); 7,38 (s, 1H); 6,58 (s, 1H); 3,97 (q, 1H);
3,68 (s, 2H); 3,50 (s, 3H); 3,01 (dd, 1H); 2,85 (dd, 1H).
13C NMR (DMSO-^6, 100 MHz): δ = 171,54; 160,80; 136,95; 128,68; 126,91; 116,40;
54,26; 30,65; 25,97; 25,11.
LC-MS: m/z = 237 ([M+H]+), 219, 193, 191, 176, 166, 164, 150, 109.
Example 13
(S) -2- (3-aminopropionylamino) -3- (3-methyl-3Jϊ-imidazol-4-yl) propionic acid
(= anserine)
To a solution of 0.20 g (0.77 mmol) (5) -2- (cyanoacetylamino) -3- (3-methyl-3H-imidazol-4-yl) propionic acid sodium salt (prepared according to Example 12) in 2 , 4 g of methanol and 1.6 g of ammonia solution (25% in Η 2 O), 16 mg of rhodium / Al 2 O 3 (5% Rh) were added. The mixture was hydrogenated at 85 ° C. and 50 bar for 1 hour. The catalyst was then filtered off. Anserine could be clearly detected in the filtrate by means of thin-layer chromatography, HPLC (by co-injection with a commercial reference substance) and LC-MS.
Gross yield: approx. 45%.
TLC: R f = 0.25 (ethyl acetate / methanol / Ammom ‘ ak H 2 O 43: 35: 8: 10).
LC-MS: m / z = 241 ([M + H] +), 224, 206, 180, 170, 126, 109.
SYN

Synthesis of L-carnosine from two amino acids β -alanine-amide and L-histidine
SYN
https://pubs.rsc.org/en/content/articlelanding/2019/cy/c9cy01622h
L-Carnosine (L-Car, β-alanyl-L-histidine) is a bioactive dipeptide with important physiological functions. Direct coupling of unprotected β-Ala (β-alanine) with L-His (L-histidine) mediated by an enzyme is a promising method for L-Car synthesis. In this study, a new recombinant dipeptidase (SmPepD) from Serratia marcescens with a high synthetic activity toward L-Car was identified by a genome mining approach and successfully expressed in Escherichia coli. Divalent metal ions strongly promoted the synthetic activity of SmPepD, with up to 21.7-fold increase of activity in the presence of 0.1 mM MnCl2. Higher temperature, lower pH and increasing substrate loadings facilitated the L-Car synthesis. Pilot biocatalytic syntheses of L-Car were performed comparatively in batch and continuous modes. In the continuous process, an ultra-filtration membrane reactor with a working volume of 5 L was employed for catalyst retention. The dipeptidase, SmPepD, showed excellent operational stability without a significant decrease in space–time yield after 4 days. The specific yield of L-Car achieved was 105 gCar gcatalyst−1 by the continuous process and 30.1 gCar gcatalyst−1 by the batch process. A nanofiltration membrane was used to isolate the desired product L-Car from the reaction mixture by selectively removing the excess substrates, β-Ala and L-His. As a result, the final L-Car content was effectively enriched from 2.3% to above 95%, which gave L-Car in 99% purity after ethanol precipitation with a total yield of 60.2%. The recovered substrate mixture of β-Ala and L-His can be easily reused, which will enable the economically attractive and environmentally benign production of the dipeptide L-Car.

-
Carnosine is a dipeptide of the amino acids beta-alanine and histidine. It is highly concentrated in muscle and brain tissues.
- [0005]
β-Alanine (or beta-alanine) is a naturally occurring beta amino acid, which is an amino acid in which the amino group is at the β-position from the carboxylate group (i.e., two atoms away).
- [0006]
β-Alanine is not used in the biosynthesis of any major proteins or enzymes. It is formed in vivo by the degradation of dihydrouracil and carnosine. It is a component of the naturally occurring peptides carnosine and anserine and also of pantothenic acid (vitamin B5), which itself is a component of coenzyme A. Under normal conditions, β-alanine is metabolized into acetic acid.
- [0007]
β-Alanine is the rate-limiting precursor of carnosine, which is to say carnosine levels are limited by the amount of available β-alanine, not histidine. Supplementation with β-alanine has been shown to increase the concentration of carnosine in muscles, decrease fatigue in athletes and increase total muscular work done.
- [0008]
Carnosine and beta-alanine are popular dietary supplements currently produced using chemical methods. Beta-alanine is also a synthetic precursor to pantothenic acid, the essential vitamin B5. Beta-alanine can also be used as a monomer for the production of a polymeric resin (U.S. Pat. No. 4,082,730).
- [0009]
Naturally, carnosine is produced exclusively in animals from beta-alanine (via uracil) and histidine. In yeasts and animals, beta-alanine is typically produced by degradation of uracil. Chemically, carnosine can be synthesized from histidine and beta-alanine derivatives. For example, the coupling of an N-(thiocarboxy) anhydride of beta-alanine with histidine has been described (Vinick et al. A simple and efficient synthesis of L-carnosine. J. Org. Chem, 1983, 48(3), pp. 392-393).
- [0010]
Beta-alanine can be produced synthetically by Michael addition of ammonia to ethyl- or methyl-acrylate. This requires the use of the caustic agent ammonia and high pressures. It is also natively produced in bacteria and yeasts in small quantities. In bacteria, beta-alanine is produced by decarboxylation of aspartate. Lysates of bacteria have been used in biocatalytic production from aspartate (Patent CN104531796A).
- [0011]
There remains a need in the industry for a safer, more economical system for the production of carnosine and beta-alanine.
- [0105]
The present disclosure provides methods for the biosynthetic production of beta-alanine and carnosine using engineered microorganisms of the present invention.
- [0106]
In one embodiment, a method of producing beta-alanine is provided. The method comprises providing a fermentation media comprising a carbon substrate, contacting said media with a recombinant yeast microorganism expressing an engineered beta-alanine biosynthetic pathway wherein said pathway comprises an aspartate to beta-alanine conversion (pathway step a), and culturing the yeast in conditions whereby beta-alanine is produced.
- [0107]
In another embodiment of the present invention, a method of producing carnosine is provided. The method comprises providing a fermentation media comprising a carbon substrate, contacting said media with a recombinant yeast microorganism expressing an engineered carnosine biosynthetic pathway wherein said pathway comprises (i) an aspartate to beta-alanine conversion (pathway step a) and (ii) a beta-alanine to carnosine conversion (pathway step b), and culturing the yeast in conditions whereby carnosine is produced.
- [0108]
In another embodiment of the present invention, a method of producing carnosine via biotransformation is provided. The method comprises providing a media comprising a carbon substrate and exogenously added beta-alanine, contacting said media with a recombinant yeast microorganism expressing an engineered carnosine biosynthetic pathway wherein said pathway comprises (i) a beta-alanine to carnosine conversion (pathway step b), and culturing the yeast in conditions whereby carnosine is produced.
- [0109]
Some embodiments of the present invention comprise yeast strains designated ca1 and ca2 and are derived from S. cerevisiae strain S288C. Each encodes at least 2 foreign genes under inducible Gal promoters. Strain ca1 also contains an additional gene, panM. The specific proteins encoded by each strain and their sequences, source, and accession numbers are provided in Table 1. The genes for these proteins are synthesized with yeast-optimized codon usage, assembled into singular genetic cassettes, and then inserted into the HO locus of S288C under URA2 selection. Strains ca1 and ca2 served as parent strains to derivatives comprising various heterologous genes. Ca2 served as a parent strain for ca7, ca8, ca9, ca10, ca11, ca12, ca14, ca15 in which the carnosine synthase is a different ortholog. Strain ca1 served as the parent strain to strains ca19, ca20, ca21, ca22, ca23, ca24, ca27, and ca28 in which the aspartate decarboxylase is a different ortholog. The specific proteins encoded by each strain and their sequences, source, and accession numbers are provided in Table 2.
- [0110]
Aspartate, histidine, and the cofactors involved in the carnosine and beta-alanine pathway are universal to all organisms, and thus the host organism could be any genetically tractable organism (plants, animals, bacteria, or fungi). Amongst yeasts, other species such as S. pombe or P. pastoris are plausible alternatives. Within the S. cerevisiae species, other strains more amenable to large-scale productions, such as CENPalpha, may be utilized.
- [0111]
The Gal promoter used in embodiments of the present invention could be replaced with constitutive promoters, or other chemically-inducible, growth phase-dependent, or stress-induced promoters. Heterologous genes of the present invention may be genomically encoded or alternatively encoded on plasmids or yeast artificial chromosomes (YACs). All genes introduced could be encoded with alternate codon usage without altering the biochemical composition of the system. All enzymes used in embodiments of the present invention have extensive orthologs in the biosphere that could be encoded as alternatives.
- [0112]
Aspartate, histidine, and the cofactors involved in this pathway are universal to all organisms, and thus the host organism could be any genetically tractable organism (plants, animals, bacteria, or fungi). Among yeast, other species such as S. pombe or P. pastoris are plausible alternatives. Within the S. cerevisiae species, other strains more amenable to large scale productions, such as CENPalpha, may be preferable. The panD gene can replaced with orthologs from other bacteria. Examples include Corynebacterium glutamicum Escherichia coli, Helicobacter pylori, Tribolium castaneum, Pectobacterium carotovorum, Actinoplanes sp. SE50/110, Taoultella ornithinolytica, Methanocaldococcus jannaschii DSM 2661 and Methanocaldococcus bathoardescens. This is shown in Table 2. Carnosine synthase is natively found in mammals, birds, and reptiles. Therefore, the chicken enzyme used in ca1 and ca2 could be replaced by various orthologs. Examples include Gorilla gorilla, Falco perefrinus, Allpiucator mississsippiensis, Ailuoropoda melanoleuca, Ursus maritimus, Python bivittatus, and Orcinus orca. This is shown in Table 2.
- Methods of Production
Culture Conditions
- [0113]
The growth medium used to test for production of carnosine by the engineered strains was Teknova SC Minimal Broth with Raffinose supplemented with 1% galactose.
- [0114]
A variety of purification protocols including solid phase extraction and cation exchange chromatography may be employed to purify the desired products from the culture supernatant or the yeast cell pellet fraction.
SYN
 |
|
| Names | |
|---|---|
| Preferred IUPAC name
(2S)-2-(3-Aminopropanamido)-3-(3H-imidazol-4-yl)propanoic acid
|
|
| Other names
β-Alanyl-L-histidine
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.610 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C9H14N4O3 | |
| Molar mass | 226.236 g·mol−1 |
| Appearance | Crystalline solid |
| Melting point | 253 °C (487 °F; 526 K) (decomposition) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Biosynthesis
Carnosine is synthesized within the body from beta-alanine and histidine. Beta-alanine is a product of pyrimidine catabolism[6] and histidine is an essential amino acid. Since beta-alanine is the limiting substrate, supplementing just beta-alanine effectively increases the intramuscular concentration of carnosine.[7][8]
Physiological effects
pH buffer
Carnosine has a pKa value of 6.83, making it a good buffer for the pH range of animal muscles.[9] Since beta-alanine is not incorporated into proteins, carnosine can be stored at relatively high concentrations (millimolar). Occurring at 17–25 mmol/kg (dry muscle),[10] carnosine (β-alanyl-L-histidine) is an important intramuscular buffer, constituting 10-20% of the total buffering capacity in type I and II muscle fibres.
Anti-oxidant
Carnosine has been proven to scavenge reactive oxygen species (ROS) as well as alpha-beta unsaturated aldehydes formed from peroxidation of cell membrane fatty acids during oxidative stress. It also buffers pH in muscle cells, and acts as a neurotransmitter in the brain. It is also a zwitterion, a neutral molecule with a positive and negative end.[citation needed]
Antiglycating
Carnosine acts as an antiglycating agent, reducing the rate of formation of advanced glycation end-products (substances that can be a factor in the development or worsening of many degenerative diseases, such as diabetes, atherosclerosis, chronic kidney failure, and Alzheimer’s disease[11]), and ultimately reducing development of atherosclerotic plaque build-up.[12][13][14]
Geroprotective
Carnosine is considered as a geroprotector.[15] Carnosine can increase the Hayflick limit in human fibroblasts,[16] as well as appearing to reduce the telomere shortening rate.[17] Carnosine may also slow aging through its anti-glycating properties (chronic glycolysis is speculated to accelerate aging).[18]
Other
Carnosine can chelate divalent metal ions.[12]
Carnosine administration has been shown to have cardioprotective properties, protecting against ischaemia-reperfusion injury, and doxorubicin-induced cardiomyopathy.[19]
Carnosine demonstrated neuroprotective effects in multiple animal studies.[20][21][22]
Research has demonstrated a positive association between muscle tissue carnosine concentration and exercise performance.[23][24][25] β-Alanine supplementation is thought to increase exercise performance by promoting carnosine production in muscle. Exercise has conversely been found to increase muscle carnosine concentrations, and muscle carnosine content is higher in athletes engaging in anaerobic exercise.[23]
Carnosine appears to protect in experimental ischemic stroke by influencing a number of mechanisms that are activated during stroke. It is a potent pH buffer and has anti matrix metalloproteinase activity, antioxidant and antiexcitotoxic properties and protects the blood brain barrier [26], [27], [28], [29], [30], [31], [32]. [33], [34], [35].
References
- ^ “C9625 L-Carnosine ~99%, crystalline”. Sigma-Aldrich.
- ^ Gulewitsch, Wl.; Amiradžibi, S. (1900). “Ueber das Carnosin, eine neue organische Base des Fleischextractes”. Berichte der Deutschen Chemischen Gesellschaft. 33 (2): 1902–1903. doi:10.1002/cber.19000330275.
- ^ Trexler, Eric T.; Smith-Ryan, Abbie E.; Stout, Jeffrey R.; Hoffman, Jay R.; Wilborn, Colin D.; Sale, Craig; Kreider, Richard B.; Jäger, Ralf; Earnest, Conrad P.; Bannock, Laurent; Campbell, Bill (2015-07-15). “International society of sports nutrition position stand: Beta-Alanine”. Journal of the International Society of Sports Nutrition. 12: 30. doi:10.1186/s12970-015-0090-y. ISSN 1550-2783. PMC 4501114. PMID 26175657.
- ^ Hipkiss, A. R. (2006). “Does chronic glycolysis accelerate aging? Could this explain how dietary restriction works?”. Annals of the New York Academy of Sciences. 1067 (1): 361–8. Bibcode:2006NYASA1067..361H. doi:10.1196/annals.1354.051. PMID 16804012. S2CID 41175541.
- ^ Alan R. Hipkiss (2009). “Chapter 3: Carnosine and Its Possible Roles in Nutrition and Health”. Advances in Food and Nutrition Research.
- ^ “beta-ureidopropionate + H2O => beta-alanine + NH4+ + CO2”. reactome. Retrieved 2020-02-08.
Cytosolic 3-ureidopropionase catalyzes the reaction of 3-ureidopropionate and water to form beta-alanine, CO2, and NH3 (van Kuilenberg et al. 2004).
- ^ Derave W, Ozdemir MS, Harris R, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E (August 9, 2007). “Beta-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters”. J Appl Physiol. 103 (5): 1736–43. doi:10.1152/japplphysiol.00397.2007. PMID 17690198. S2CID 6990201.
- ^ Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007). “Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity”. Amino Acids. 32 (2): 225–33. doi:10.1007/s00726-006-0364-4. PMID 16868650. S2CID 23988054.
- ^ Bate-Smith, EC (1938). “The buffering of muscle in rigor: protein, phosphate and carnosine”. Journal of Physiology. 92 (3): 336–343. doi:10.1113/jphysiol.1938.sp003605. PMC 1395289. PMID 16994977.
- ^ Mannion, AF; Jakeman, PM; Dunnett, M; Harris, RC; Willan, PLT (1992). “Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans”. Eur. J. Appl. Physiol. 64 (1): 47–50. doi:10.1007/BF00376439. PMID 1735411. S2CID 24590951.
- ^ Vistoli, G; De Maddis, D; Cipak, A; Zarkovic, N; Carini, M; Aldini, G (Aug 2013). “Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation”. Free Radic. Res. 47: Suppl 1:3–27. doi:10.3109/10715762.2013.815348. PMID 23767955. S2CID 207517855.
- ^ Jump up to:a b Reddy, V. P.; Garrett, MR; Perry, G; Smith, MA (2005). “Carnosine: A Versatile Antioxidant and Antiglycating Agent”. Science of Aging Knowledge Environment. 2005 (18): pe12. doi:10.1126/sageke.2005.18.pe12. PMID 15872311.
- ^ Rashid, Imran; Van Reyk, David M.; Davies, Michael J. (2007). “Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro”. FEBS Letters. 581 (5): 1067–70. doi:10.1016/j.febslet.2007.01.082. PMID 17316626. S2CID 46535145.
- ^ Hipkiss, A. R. (2005). “Glycation, ageing and carnosine: Are carnivorous diets beneficial?”. Mechanisms of Ageing and Development. 126 (10): 1034–9. doi:10.1016/j.mad.2005.05.002. PMID 15955546. S2CID 19979631.
- ^ Boldyrev, A. A.; Stvolinsky, S. L.; Fedorova, T. N.; Suslina, Z. A. (2010). “Carnosine as a natural antioxidant and geroprotector: From molecular mechanisms to clinical trials”. Rejuvenation Research. 13 (2–3): 156–8. doi:10.1089/rej.2009.0923. PMID 20017611.
- ^ McFarland, G; Holliday, R (1994). “Retardation of the Senescence of Cultured Human Diploid Fibroblasts by Carnosine”. Experimental Cell Research. 212 (2): 167–75. doi:10.1006/excr.1994.1132. PMID 8187813.
- ^ Shao, Lan; Li, Qing-Huan; Tan, Zheng (2004). “L-Carnosine reduces telomere damage and shortening rate in cultured normal fibroblasts”. Biochemical and Biophysical Research Communications. 324 (2): 931–6. doi:10.1016/j.bbrc.2004.09.136. PMID 15474517.
- ^ Hipkiss, A. R. (2006). “Does Chronic Glycolysis Accelerate Aging? Could This Explain How Dietary Restriction Works?”. Annals of the New York Academy of Sciences. 1067 (1): 361–8. Bibcode:2006NYASA1067..361H. doi:10.1196/annals.1354.051. PMID 16804012. S2CID 41175541.
- ^ McCarty, Mark F; DiNicolantonio, James J (2014-08-04). “β-Alanine and orotate as supplements for cardiac protection”. Open Heart. 1 (1): e000119. doi:10.1136/openhrt-2014-000119. ISSN 2053-3624. PMC 4189254. PMID 25332822.
- ^ Virdi, Jasleen Kaur; Bhanot, Amritansh; Jaggi, Amteshwar Singh; Agarwal, Neha (2020-10-02). “Investigation on beneficial role of l -carnosine in neuroprotective mechanism of ischemic postconditioning in mice: possible role of histidine histamine pathway”. International Journal of Neuroscience. 130 (10): 983–998. doi:10.1080/00207454.2020.1715393. ISSN 0020-7454. PMID 31951767. S2CID 210710039.
- ^ Rajanikant, G.K.; Zemke, Daniel; Senut, Marie-Claude; Frenkel, Mark B.; Chen, Alex F.; Gupta, Rishi; Majid, Arshad (November 2007). “Carnosine Is Neuroprotective Against Permanent Focal Cerebral Ischemia in Mice”. Stroke. 38 (11): 3023–3031. doi:10.1161/STROKEAHA.107.488502. ISSN 0039-2499. PMID 17916766.
- ^ Min, Jiangyong; Senut, Marie-Claude; Rajanikant, Krishnamurthy; Greenberg, Eric; Bandagi, Ram; Zemke, Daniel; Mousa, Ahmad; Kassab, Mounzer; Farooq, Muhammad U.; Gupta, Rishi; Majid, Arshad (October 2008). “Differential neuroprotective effects of carnosine, anserine, and N -acetyl carnosine against permanent focal ischemia”. Journal of Neuroscience Research. 86 (13): 2984–2991. doi:10.1002/jnr.21744. PMC 2805719. PMID 18543335.
- ^ Jump up to:a b Culbertson, Julie Y.; Kreider, Richard B.; Greenwood, Mike; Cooke, Matthew (2010-01-25). “Effects of Beta-Alanine on Muscle Carnosine and Exercise Performance:A Review of the Current Literature”. Nutrients. 2 (1): 75–98. doi:10.3390/nu2010075. ISSN 2072-6643. PMC 3257613. PMID 22253993.
- ^ Baguet, Audrey; Bourgois, Jan; Vanhee, Lander; Achten, Eric; Derave, Wim (2010-07-29). “Important role of muscle carnosine in rowing performance”. Journal of Applied Physiology. 109 (4): 1096–1101. doi:10.1152/japplphysiol.00141.2010. ISSN 8750-7587. PMID 20671038.
- ^ Varanoske, Alyssa N.; Hoffman, Jay R.; Church, David D.; Wang, Ran; Baker, Kayla M.; Dodd, Sarah J.; Coker, Nicholas A.; Oliveira, Leonardo P.; Dawson, Virgil L.; Fukuda, David H.; Stout, Jeffrey R. (2017-09-07). “Influence of Skeletal Muscle Carnosine Content on Fatigue during Repeated Resistance Exercise in Recreationally Active Women”. Nutrients. 9 (9): 988. doi:10.3390/nu9090988. ISSN 2072-6643. PMC 5622748. PMID 28880219.
26. Kim EH, Kim ES, Shin D, Kim D, Choi S, Shin YJ, Kim KA, Noh D, Caglayan AB, Rajanikant GK, Majid A, Bae ON. Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases. Int J Mol Sci. 2021 Jul 13;22(14):7495. doi: 10.3390/ijms22147495. PMID: 34299128; PMCID: PMC8306548.
27. Jain S, Kim ES, Kim D, Burrows D, De Felice M, Kim M, Baek SH, Ali A, Redgrave J, Doeppner TR, Gardner I, Bae ON, Majid A. Comparative Cerebroprotective Potential of d- and l-Carnosine Following Ischemic Stroke in Mice. Int J Mol Sci. 2020 Apr 26;21(9):3053. doi: 10.3390/ijms21093053. PMID: 32357505; PMCID: PMC7246848.
28. Kim ES, Kim D, Nyberg S, Poma A, Cecchin D, Jain SA, Kim KA, Shin YJ, Kim EH, Kim M, Baek SH, Kim JK, Doeppner TR, Ali A, Redgrave J, Battaglia G, Majid A, Bae ON. LRP-1 functionalized polymersomes enhance the efficacy of carnosine in experimental stroke. Sci Rep. 2020 Jan 20;10(1):699. doi: 10.1038/s41598-020-57685-5. PMID: 31959846; PMCID: PMC6971073.
29. Schön M, Mousa A, Berk M, Chia WL, Ukropec J, Majid A, Ukropcová B, de Courten B. The Potential of Carnosine in Brain-Related Disorders: A Comprehensive Review of Current Evidence. Nutrients. 2019 May 28;11(6):1196. doi: 10.3390/nu11061196. PMID: 31141890; PMCID: PMC6627134.
30. Davis CK, Laud PJ, Bahor Z, Rajanikant GK, Majid A. Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J Cereb Blood Flow Metab. 2016 Oct;36(10):1686-1694. doi: 10.1177/0271678X16658302. Epub 2016 Jul 8. PMID: 27401803; PMCID: PMC5046161.
31. Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014 Aug;45(8):2438-2443. doi: 10.1161/STROKEAHA.114.005183. Epub 2014 Jun 17. PMID: 24938837; PMCID: PMC4211270.
32. Bae ON, Majid A. Role of histidine/histamine in carnosine-induced neuroprotection during ischemic brain damage. Brain Res. 2013 Aug 21;1527:246-54. doi: 10.1016/j.brainres.2013.07.004. Epub 2013 Jul 11. PMID: 23850642.
33. Bae ON, Serfozo K, Baek SH, Lee KY, Dorrance A, Rumbeiha W, Fitzgerald SD, Farooq MU, Naravelta B, Bhatt A, Majid A. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013 Jan;44(1):205-12. doi: 10.1161/STROKEAHA.112.673954. Epub 2012 Dec 18. PMID: 23250994; PMCID: PMC3678096.
34. Min J, Senut MC, Rajanikant K, Greenberg E, Bandagi R, Zemke D, Mousa A, Kassab M, Farooq MU, Gupta R, Majid A. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res. 2008 Oct;86(13):2984-91. doi: 10.1002/jnr.21744. PMID: 18543335; PMCID: PMC2805719.
35. Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, Majid A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007 Nov;38(11):3023-31. doi: 10.1161/STROKEAHA.107.488502. Epub 2007 Oct 4. PMID: 17916766.
////////L-CARNOSINE, カルノシン , b-Alanyl-L-histidine, ignotine, 8HO6PVN24W, カルノシン , Dragosine, Ignotin, Ignotine, Karnozin, L-Carnosine, N-(β-Alanyl)-L-histidine, NSC 524045, Sevitin, β-Alanylhistidine