LIDOCAINE

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
CLIP
https://people.chem.umass.edu/mcdaniel/CHEM-267/Experiments/Lidocaine.pdf
https://pubs.acs.org/doi/10.1021/ed076p1557
http://www.asianjournalofchemistry.co.in/User/ViewFreeArticle.aspx?ArticleID=19_7_12
PATENT
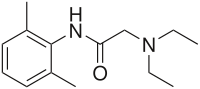
1H NMR
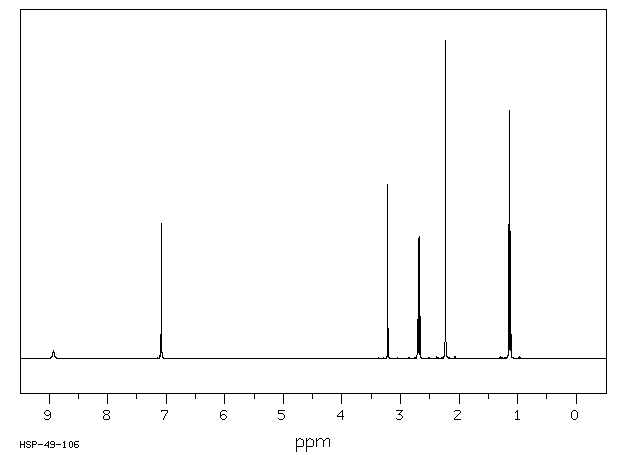
13C NMR
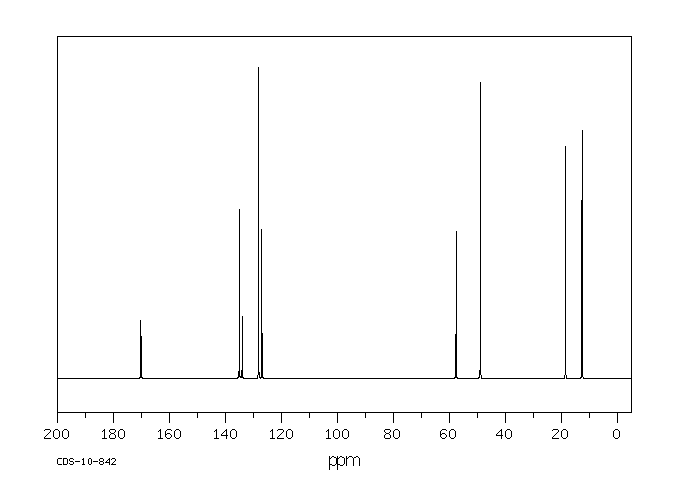
MS
IR KBR

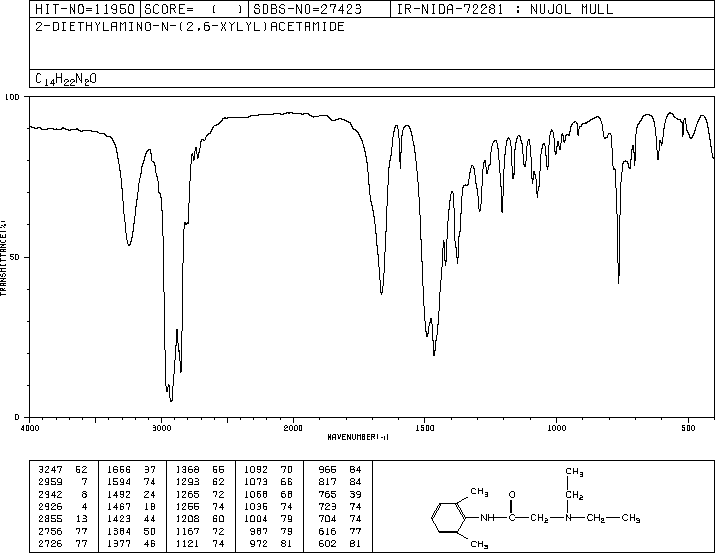
Lidocaine is an antiarrhythmic medicine and also serves as a local anaesthetic drug. It is utilized in topical application to relieve pain, burning and itching sensation caused from skin inflammations. This drug is mainly used for minor surgeries. Figure 1 shows the 1H NMR spectrum of 200 mM lidocaine in CDCl3.
.jpg)
Figure 1. Proton NMR spectrum of 200 mM lidocaine in CDCl3.
1H NMR Relaxation
Figures 2, 3 and 4 show the relaxation time measurements. It can be seen that the relaxation times are shortest for the CH2 protons and longest for the CH protons. The first data point amplitude increases with the number of protons for the related peak.
.jpg)
Figure 2. Proton T1 relaxation time measurement of 200 mM lidocaine in CDCl3.
.jpg)
Figure 3. Proton T2 relaxation time measurement of 200 mM lidocaine in CDCl3.
.jpg)
Figure 4. COSY spectrum of 200 mM lidocaine in CDCl3. The cross-peaks and corresponding exchanging protons are labeled by colour-coded arrows and ellipses.
2D COSY
Figure 4 shows the 2D COSY spectrum where two spin systems (6,7,8) to (10,11) can be clearly seen. For instance, the methyl groups at 10 and 11 positions bond to aromatic protons at 6 and 8 positions, while the methyl groups at 16 and 17 positions bond to the ethylene groups at 14 and 15 positions. No coupling occurs at positions (6,7,8) to (16,17) or (14,15).
2D Homonuclear J-Resolved Spectroscopy
The chemical shift in the 2D homonuclear j-resolved spectrum appears along the direct (f2) direction and the effects of coupling between protons appear along the indirect (f1) dimension. This enables the assignment of chemical shifts of multiplets and may help in measuring unresolved couplings. Also, a decoupled 1D proton spectrum is produced by the projection along the f1 dimension. The 2D homonuclear j-resolved spectrum of lidocaine, plus the 1D proton spectrum (blue line) are shown in Figure 5.
.jpg)
Figure 5. Homonuclear j-resolved spectrum of 200 mM lidocaine in CDCl3. The multiplet splitting frequencies for different couplings are colour- coded.
The projection which is vertical reveals how the multiplets disintegrate into a single peak, which makes the 1D spectrum more simplified. Peak multiplicities are produced by vertical traces from peaks in the 2D spectrum and help in determining the frequencies of proton-proton coupling. When coupling frequencies are compared between different peaks, information can be obtained regarding which peaks are bonded to each other. Also, Information regarding the coupling strength can be obtained from the size of the coupling frequencies. These couplings substantiate the results of the COSY experiment.
However, in this experiment, the effects of second order coupling appear in the f1 direction as additional peaks which are equidistant from the coupling partners detached from the zero frequency in the f1 dimension. These peaks provide proof of second order coupling partners, but are generally considered as artifacts. Figure 6 shows these coupling partners and additional peaks marked by colour-coded arrows and ellipses.
.jpg)
Figure 6. Homonuclear j-resolved spectrum of 200 mM lidocaine in CDCl3 showing the extra peaks due to strong couplings.
1D 13C Spectra
Figure 7 shows the 13C NMR spectra of 1 M lidocaine in CDCl3. Since the 1D Carbon experiment is highly susceptible to the 13C nuclei in the specimen, it easily and clearly resolves 9 resonances. In this experiment, only carbons coupled to protons are seen.
.jpg)
Figure 7. Carbon spectra of 1 M lidocaine in CDCl3.
Given the fact that the DEPT spectra do not display the peaks at 170 and 135ppm, they must be part of quaternary carbons. The DEPT-135 and the DEPT-45 experiments provide signals of CH3, CH2 and CH groups, while the DEPT-90 experiment provides only the signal of CH groups. However, in DEPT-135 the CH2 groups occur as negative peaks. It can thus be summed up that the peaks between 45 and 60ppm belong to ethylene groups; the peaks between 10 and 20ppm are part of the methyl groups; and the peaks between 125 and 130ppm belong to methyne groups. A similar study can be carried out on the C and CH peaks.
Heteronuclear Correlation
The Heteronuclear Correlation (HETCOR) experiment identifies the proton signal that appears along the indirect dimension and the carbon signal along the direct dimension. Figure 8 shows the HETCOR spectrum of 1 M lidocaine in CDCl3. in the 2D spectrum, the peaks reveal which proton is attached to which carbon. This experiment helps in resolving assignment uncertainty from the ID carbon spectra.
.jpg)
Figure 8. HETCOR spectrum of 1 M lidocaine in CDCl3.
Heteronuclear Multiple Quantum Coherence
Heteronuclear Multiple Quantum Coherence (HMQC) is similar to the HETCOR experiment and is utilized to associate proton resonances to the carbons that are coupled directly to those protons. But in the HMQC experiment, the proton signal appears along the direct dimension and the carbon signal along the indirect dimension. Figure 9 shows the HMQC spectrum of 1 M lidocaine in CDCl3. In the 2D spectrum, the peaks show which proton is attached to which carbon. For conclusive peak assignment, a similar study with the HETCOR spectrum can be carried out.
.jpg)
Figure 9. HMQC spectrum of 1 M lidocaine in CDCl3.
Heteronuclear Multiple Bond Correlation
The Heteronuclear Multiple Bond Correlation (HMBC) experiment can be employed to achieve long-range correlations of proton and carbon via two or three bond couplings. Similar to the HMQC experiment, the proton signal appears along the direct dimension and the carbon signal along the indirect dimension. Figure 10 shows the HMBC spectrum of 1 M lidocaine in CDCl3.
.jpg)
Figure 10. HMBC spectrum of 1 M lidocaine in CDCl3, with some of the long-range couplings marked.
The couplings amid the molecular positions appear analogous to the couplings seen in the COSY spectrum; however, the HMBC also displays couplings to quaternary carbons, which are not seen either in HMQC or COSY experiments. In addition, there is a correlation between protons and carbons. This is attributed to three-bond bonding from 14 and 15 and vice versa, as shown in light green in Figure 1.
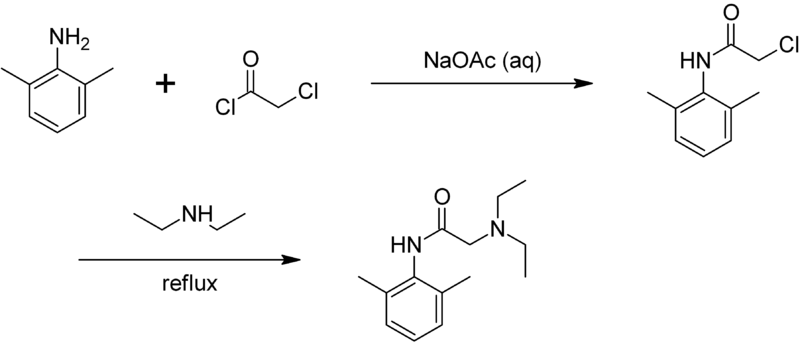
SYN
Synthesis of lidocaine T. J. Reilly (1999). “The Preparation of Lidocaine”. J. Chem. Ed. 76 (11): 1557.
CLIP
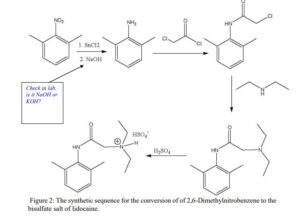
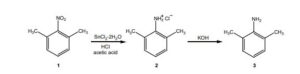
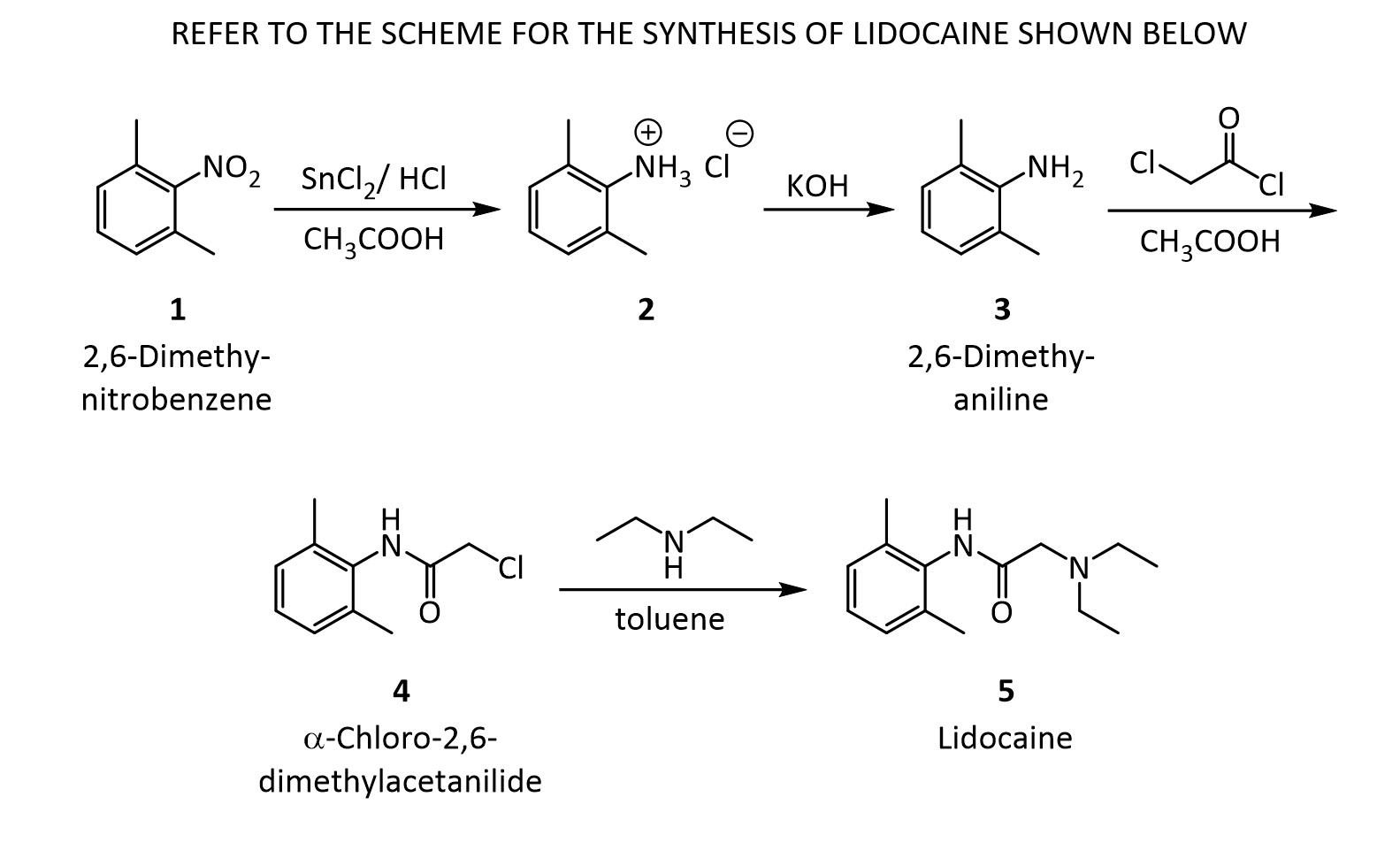
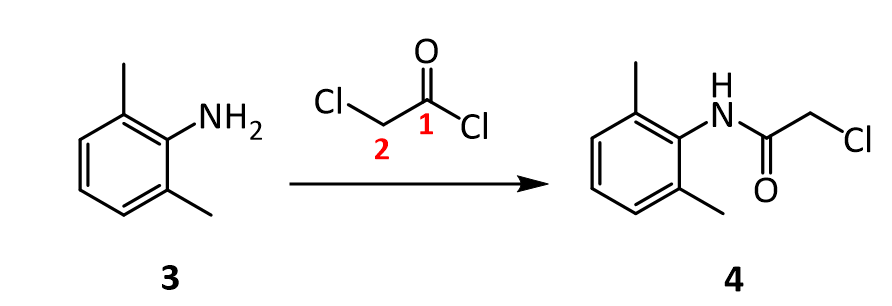
The Present Synthesis Of Lidocaine Begins With 2,6-Dimethylnitrobenzene (1). This Compound Can Be Made From 1,3-Dimethylbenzene, Also Known As M-Xylene, Which Is More Difficult To Make. Luckily,
This problem has been solved!
- The present synthesis of lidocaine begins with 2,6-dimethylnitrobenzene (1). This compound can be made from 1,3-dimethylbenzene, also known as m-xylene, which is more difficult to make. Luckily, m-xylene is commercially available, so a synthesis of 1 from m-xylene is a practical alternative if one wants to begin the synthesis of lidocaine with m-xylene. Suppose you want to prepare 1 from m-xylene. Show with chemical equations the reagents that you would use, and the possible isomers that would result.
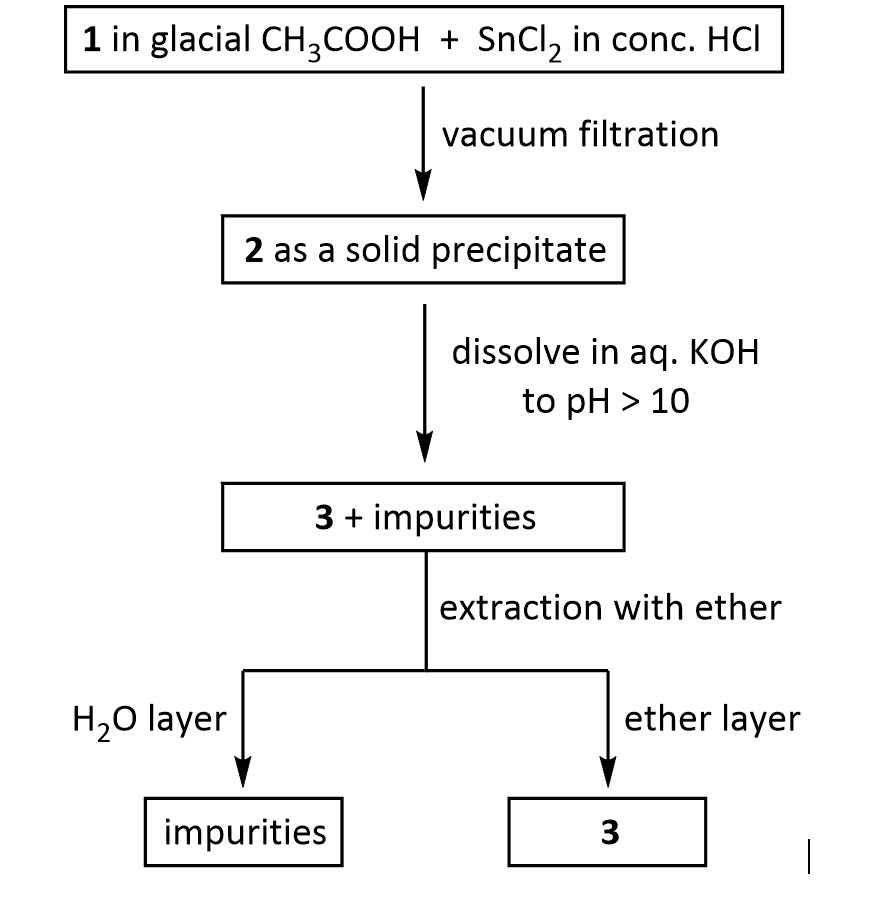
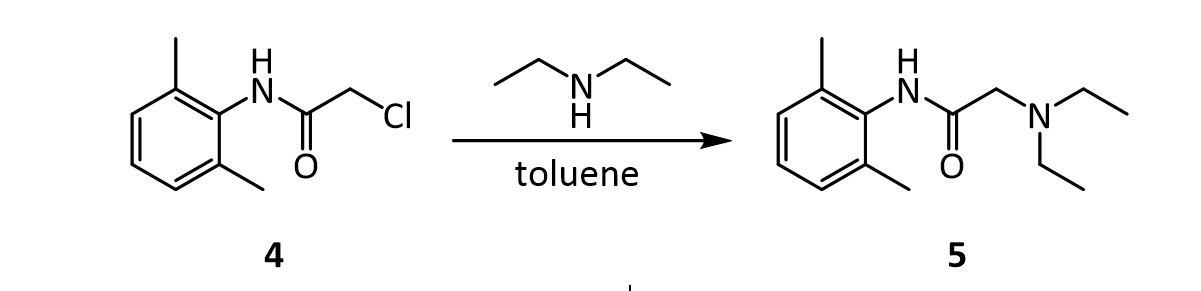
2. The practical transformation of 1 into 3 is carried out by the following scheme:
Suppose you dissolve the solid precipitate of 2 in water, but forget to include the KOH in the second step above. What would happen after the extraction with ether? Give your answer in terms of what would be found in the ether layer, and in the aqueous layer.
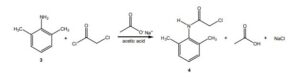
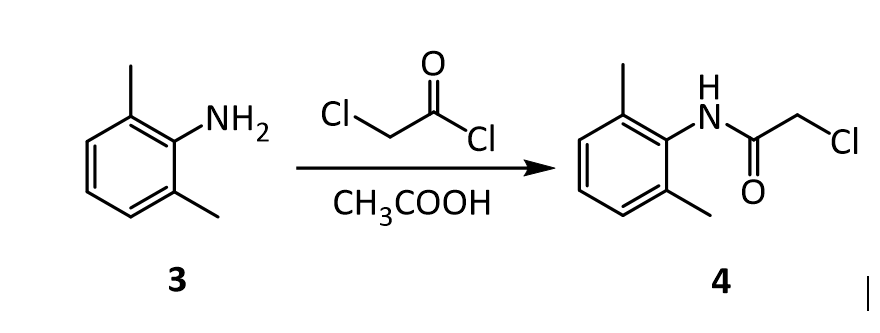
3. Suppose you’re out of acetic acid (CH3COOH) and decide to use ethanol (CH3 CH2OH) as the solvent in the transformation of 3 into 4. Would this be a wise choice, and why?
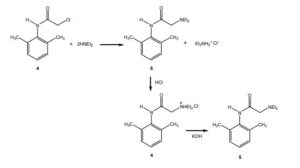
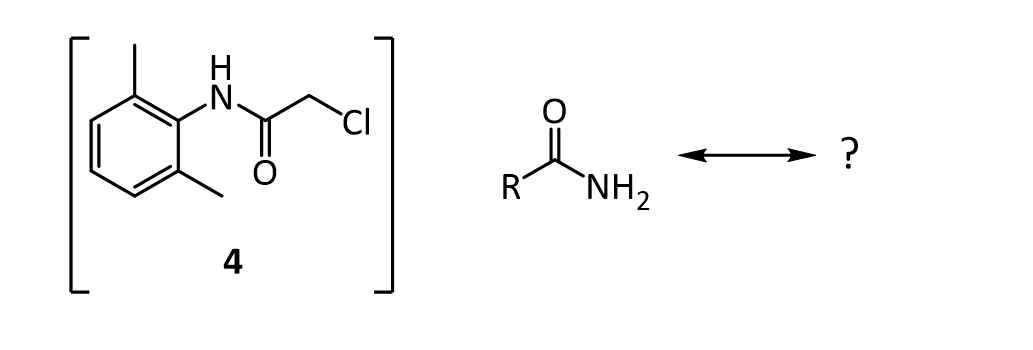
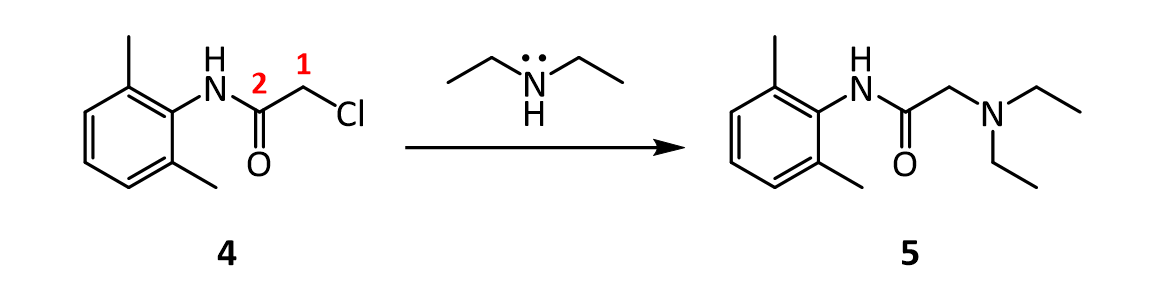
4.The amide 4 has a nitrogen attached to the benzene ring, and a chlorine attached to a primary carbon. Yet, it doesn’t react with itself in a nucleophilic displacement. Why is the nitrogen in the amide not nucleophilic? Give your answer in terms of the resonance forms of amides in general:
5. In the reaction below, what factors come into play to favor attack of the aniline 3 on the carbonyl carbon of the acid chloride (carbon 1 in red), rather than at the a-carbon (carbon 2 in red)?
6. Before carrying out the transformation below, compound 4 and the glassware used must be oven-dried. What would happen if the reaction was attempted using wet 4?
7.In the reaction below, what factors come into play to favor attack of diethylamine on the a-carbon (carbon 1 in red), rather than on the amide C=O carbon (carbon 2 in red)?
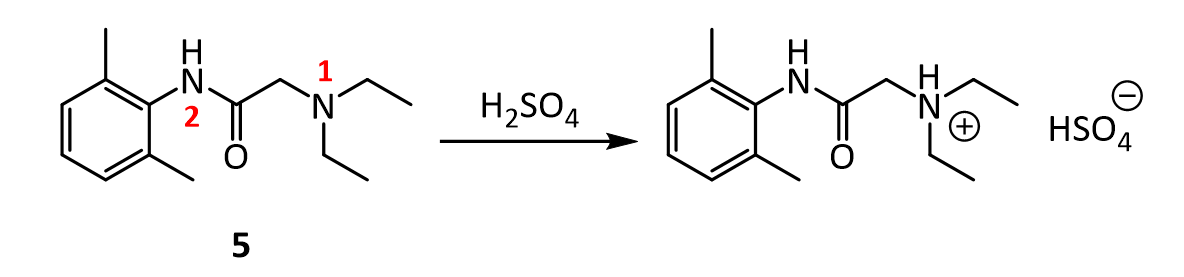
8. In the reaction below, why does the amine nitrogen (#1 in red) undergo protonation with H2SO4 preferentially over the amide nitrogen (#2 in red)? In other words, why is nitrogen 1 basic, but nitrogen 2 is not?
9.Lidocaine and other drugs containing amino groups are usually marketed as their hydrochloride or hydrogen sulfate salts, rather than as “free amines.” Provide two reasons why this practice makes sense.
10.Although lidocaine is marketed as its hydrochloride salt, it doesn’t exhibit the same level of physiological activity as the free amine. The free amine is more lipophilic and diffuses across a neuron cell membrane more rapidly than the ionic salt, resulting in a more rapid onset of anesthesia. Therefore, sodium bicarbonate (NaHCO3) is added to a solution of lidocaine prior to injection. How does the addition of sodium bicarbonate promote a faster anesthetic effect?
CLIP
https://www.cpp.edu/~lsstarkey/courses/CHM-Lab/LidocaineRemoteHandout.pdf
CLIP
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia.[7][8] When used for local anaesthesia or in nerve blocks, lidocaine typically begins working within several minutes and lasts for half an hour to three hours.[8][9] Lidocaine mixtures may also be applied directly to the skin or mucous membranes to numb the area.[8] It is often used mixed with a small amount of adrenaline (epinephrine) to prolong its local effects and to decrease bleeding.[8]
If injected intravenously, it may cause cerebral effects such as confusion, changes in vision, numbness, tingling, and vomiting.[7] It can cause low blood pressure and an irregular heart rate.[7] There are concerns that injecting it into a joint can cause problems with the cartilage.[8] It appears to be generally safe for use in pregnancy.[7] A lower dose may be required in those with liver problems.[7] It is generally safe to use in those allergic to tetracaine or benzocaine.[8] Lidocaine is an antiarrhythmic medication of the class Ib type.[7] This means it works by blocking sodium channels and thus decreasing the rate of contractions of the heart.[7] When injected near nerves, the nerves cannot conduct signals to or from the brain.[8]
Lidocaine was discovered in 1946 and went on sale in 1948.[10] It is on the World Health Organization’s List of Essential Medicines.[11] It is available as a generic medication.[8][12] In 2018, it was the 233rd most commonly prescribed medication in the United States, with more than 2 million prescriptions.[13][14]
Medical uses
Local numbing agent
The efficacy profile of lidocaine as a local anaesthetic is characterized by a rapid onset of action and intermediate duration of efficacy. Therefore, lidocaine is suitable for infiltration, block, and surface anaesthesia. Longer-acting substances such as bupivacaine are sometimes given preference for spinal and epidural anaesthesias; lidocaine, though, has the advantage of a rapid onset of action. Adrenaline vasoconstricts arteries, reducing bleeding and also delaying the resorption of lidocaine, almost doubling the duration of anaesthesia.
Lidocaine is one of the most commonly used local anaesthetics in dentistry. It can be administered in multiple ways, most often as a nerve block or infiltration, depending on the type of treatment carried out and the area of the mouth worked on.[15]
For surface anaesthesia, several formulations can be used for endoscopies, before intubations, etc. Buffering the pH of lidocaine makes local numbing less painful.[16] Lidocaine drops can be used on the eyes for short ophthalmic procedures. There is tentative evidence for topical lidocaine for neuropathic pain and skin graft donor site pain.[17][18] As a local numbing agent, it is used for the treatment of premature ejaculation.[19]
An adhesive transdermal patch containing a 5% concentration of lidocaine in a hydrogel bandage, is approved by the US FDA for reducing nerve pain caused by shingles.[20] The transdermal patch is also used for pain from other causes, such as compressed nerves and persistent nerve pain after some surgeries.
Heart arrhythmia
Lidocaine is also the most important class-1b antiarrhythmic drug; it is used intravenously for the treatment of ventricular arrhythmias (for acute myocardial infarction, digoxin poisoning, cardioversion, or cardiac catheterization) if amiodarone is not available or contraindicated. Lidocaine should be given for this indication after defibrillation, CPR, and vasopressors have been initiated. A routine preventive dose is no longer recommended after a myocardial infarction as the overall benefit is not convincing.[21]
Epilepsy
A 2013 review on treatment for neonatal seizures recommended intravenous lidocaine as a second-line treatment, if phenobarbital fails to stop seizures.[22]
Other
Intravenous lidocaine infusions are also used to treat chronic pain and acute surgical pain as an opiate sparing technique. The quality of evidence for this use is poor so it is difficult to compare it to placebo or an epidural.[23]
Inhaled lidocaine can be used as a cough suppressor acting peripherally to reduce the cough reflex. This application can be implemented as a safety and comfort measure for patients who have to be intubated, as it reduces the incidence of coughing and any tracheal damage it might cause when emerging from anaesthesia.[24]
Lidocaine, along with ethanol, ammonia, and acetic acid, may also help in treating jellyfish stings, both numbing the affected area and preventing further nematocyst discharge.[25][26]
For gastritis, drinking a viscous lidocaine formulation may help with the pain.[27]
Adverse effects
Adverse drug reactions (ADRs) are rare when lidocaine is used as a local anesthetic and is administered correctly. Most ADRs associated with lidocaine for anesthesia relate to administration technique (resulting in systemic exposure) or pharmacological effects of anesthesia, and allergic reactions only rarely occur.[28] Systemic exposure to excessive quantities of lidocaine mainly result in central nervous system (CNS) and cardiovascular effects – CNS effects usually occur at lower blood plasma concentrations and additional cardiovascular effects present at higher concentrations, though cardiovascular collapse may also occur with low concentrations. ADRs by system are:
- CNS excitation: nervousness, agitation, anxiety, apprehension, tingling around the mouth (circumoral paraesthesia), headache, hyperesthesia, tremor, dizziness, pupillary changes, psychosis, euphoria, hallucinations, and seizures
- CNS depression with increasingly heavier exposure: drowsiness, lethargy, slurred speech, hypoesthesia, confusion, disorientation, loss of consciousness, respiratory depression and apnoea.
- Cardiovascular: hypotension, bradycardia, arrhythmias, flushing, venous insufficiency, increased defibrillator threshold, edema, and/or cardiac arrest – some of which may be due to hypoxemia secondary to respiratory depression.[29]
- Respiratory: bronchospasm, dyspnea, respiratory depression or arrest
- Gastrointestinal: metallic taste, nausea, vomiting
- Ears: tinnitus
- Eyes: local burning, conjunctival hyperemia, corneal epithelial changes/ulceration, diplopia, visual changes (opacification)
- Skin: itching, depigmentation, rash, urticaria, edema, angioedema, bruising, inflammation of the vein at the injection site, irritation of the skin when applied topically
- Blood: methemoglobinemia
- Allergy
ADRs associated with the use of intravenous lidocaine are similar to toxic effects from systemic exposure above. These are dose-related and more frequent at high infusion rates (≥3 mg/min). Common ADRs include: headache, dizziness, drowsiness, confusion, visual disturbances, tinnitus, tremor, and/or paraesthesia. Infrequent ADRs associated with the use of lidocaine include: hypotension, bradycardia, arrhythmias, cardiac arrest, muscle twitching, seizures, coma, and/or respiratory depression.[29]
It is generally safe to use lidocaine with vasoconstrictor such as adrenaline, including in regions such as the nose, ears, fingers, and toes.[30] While concerns of tissue death if used in these areas have been raised, evidence does not support these concerns.[30]
Interactions
Any drugs that are also ligands of CYP3A4 and CYP1A2 can potentially increase serum levels and potential for toxicity or decrease serum levels and the efficacy, depending on whether they induce or inhibit the enzymes, respectively. Drugs that may increase the chance of methemoglobinemia should also be considered carefully. Dronedarone and liposomal morphine are both absolutely a contraindication, as they may increase the serum levels, but hundreds of other drugs require monitoring for interaction.[31]
Contraindications
Absolute contraindications for the use of lidocaine include:
- Heart block, second or third degree (without pacemaker)
- Severe sinoatrial block (without pacemaker)
- Serious adverse drug reaction to lidocaine or amide local anesthetics
- Hypersensitivity to corn and corn-related products (corn-derived dextrose is used in the mixed injections)
- Concurrent treatment with quinidine, flecainide, disopyramide, procainamide (class I antiarrhythmic agents)
- Prior use of amiodarone hydrochloride
- Adams-Stokes syndrome[32]
- Wolff-Parkinson-White syndrome[32]
- Lidocaine viscous is not recommended by the FDA to treat teething pain in children and infants.[33]
Exercise caution in patients with any of these:
- Hypotension not due to arrhythmia
- Bradycardia
- Accelerated idioventricular rhythm
- Elderly patients
- Ehlers-Danlos Syndrome
- Pseudocholinesterase deficiency
- Intra-articular infusion (this is not an approved indication and can cause chondrolysis)
- Porphyria, especially acute intermittent porphyria; lidocaine has been classified as porphyrogenic because of the hepatic enzymes it induces,[34] although clinical evidence suggests it is not.[35] Bupivacaine is a safe alternative in this case.
- Impaired liver function – people with lowered hepatic function may have an adverse reaction with repeated administration of lidocaine because the drug is metabolized by the liver. Adverse reactions may include neurological symptoms (e.g. dizziness, nausea, muscle twitches, vomiting, or seizures).[36]
Overdosage
Overdoses of lidocaine may result from excessive administration by topical or parenteral routes, accidental oral ingestion of topical preparations by children (who are more susceptible to overdose), accidental intravenous (rather than subcutaneous, intrathecal, or paracervical) injection, or from prolonged use of subcutaneous infiltration anesthesia during cosmetic surgery.
Such overdoses have often led to severe toxicity or death in both children and adults. Lidocaine and its two major metabolites may be quantified in blood, plasma, or serum to confirm the diagnosis in potential poisoning victims or to assist forensic investigation in a case of fatal overdose.
Lidocaine is often given intravenously as an antiarrhythmic agent in critical cardiac-care situations.[37] Treatment with intravenous lipid emulsions (used for parenteral feeding) to reverse the effects of local anaesthetic toxicity is becoming more common.[38]
Postarthroscopic glenohumeral chondrolysis
Lidocaine in large amounts may be toxic to cartilage and intra-articular infusions can lead to postarthroscopic glenohumeral chondrolysis.[39]
Pharmacology
Mechanism of action
Lidocaine alters signal conduction in neurons by prolonging the inactivation of the fast voltage-gated Na+ channels in the neuronal cell membrane responsible for action potential propagation.[40] With sufficient blockage, the voltage-gated sodium channels will not open and an action potential will not be generated. Careful titration allows for a high degree of selectivity in the blockage of sensory neurons, whereas higher concentrations also affect other types of neurons.
The same principle applies for this drug’s actions in the heart. Blocking sodium channels in the conduction system, as well as the muscle cells of the heart, raises the depolarization threshold, making the heart less likely to initiate or conduct early action potentials that may cause an arrhythmia.[41]
Pharmacokinetics
When used as an injectable it typically begins working within four minutes and lasts for half an hour to three hours.[8][9] Lidocaine is about 95% metabolized (dealkylated) in the liver mainly by CYP3A4 to the pharmacologically active metabolites monoethylglycinexylidide (MEGX) and then subsequently to the inactive glycine xylidide. MEGX has a longer half-life than lidocaine, but also is a less potent sodium channel blocker.[42] The volume of distribution is 1.1 L/kg to 2.1 L/kg, but congestive heart failure can decrease it. About 60% to 80% circulates bound to the protein alpha1 acid glycoprotein. The oral bioavailability is 35% and the topical bioavailability is 3%.
The elimination half-life of lidocaine is biphasic and around 90 min to 120 min in most patients. This may be prolonged in patients with hepatic impairment (average 343 min) or congestive heart failure (average 136 min).[43] Lidocaine is excreted in the urine (90% as metabolites and 10% as unchanged drug).[44]
History
Lidocaine, the first amino amide–type local anesthetic, was first synthesized under the name ‘xylocaine’ by Swedish chemist Nils Löfgren in 1943.[45][46][47] His colleague Bengt Lundqvist performed the first injection anesthesia experiments on himself.[45] It was first marketed in 1949.
Society and culture
Dosage forms
Lidocaine, usually in the form of its hydrochloride salt, is available in various forms including many topical formulations and solutions for injection or infusion.[48] It is also available as a transdermal patch, which is applied directly to the skin.
Names
Lidocaine is the International Nonproprietary Name (INN), British Approved Name (BAN), and Australian Approved Name (AAN),[49] while lignocaine is the former BAN[citation needed] and AAN. Both the old and new names will be displayed on the product label in Australia until at least 2023.[50]
Xylocaine is a brand name.
Recreational use
As of 2021, lidocaine is not listed by the World Anti-Doping Agency as a substance whose use is banned in sport.[51] It is used as an adjuvant, adulterant, and diluent to street drugs such as cocaine and heroin.[52] It is one of the three common ingredients in site enhancement oil used by bodybuilders.[53]
Adulterant in cocaine
Lidocaine is often added to cocaine as a diluent.[54][55] Cocaine and lidocaine both numb the gums when applied. This gives the user the impression of high-quality cocaine, when in actuality the user is receiving a diluted product.[56]
Compendial status
Veterinary use
It is a component of the veterinary drug Tributame along with embutramide and chloroquine used to carry out euthanasia on horses and dogs.[58][59]
References
- ^ “Lidocaine”. Merriam-Webster Dictionary.
- ^ “Lidocaine”. Dictionary.com Unabridged. Random House.
- ^ “Poisons Standard February 2021”. Federal Register of Legislation. 1 January 2021. Retrieved 11 April 2021.
- ^ “Lidocaine Hydrochloride Injection BP 1% w/v – Summary of Product Characteristics (SmPC)”. (emc). 29 June 2020. Retrieved 11 April 2021.
- ^ “Xylocaine MPF- lidocaine hydrochloride injection, solution Xylocaine- lidocaine hydrochloride injection, solution Xylocaine- lidocaine hydrochloride,epinephrine bitartrate injection, solution”. DailyMed. Retrieved 11 April 2021.
- ^ “Ztlido- lidocaine patch”. DailyMed. Retrieved 11 April 2021.
- ^ Jump up to:a b c d e f g h i j k “Lidocaine Hydrochloride (Antiarrhythmic)”. The American Society of Health-System Pharmacists. Archivedfrom the original on 2015-08-10. Retrieved Aug 26, 2015.
- ^ Jump up to:a b c d e f g h i j “Lidocaine Hydrochloride (Local)”. The American Society of Health-System Pharmacists. Archived from the original on 2015-09-06. Retrieved Aug 26, 2015.
- ^ Jump up to:a b c J. P. Nolan; P. J. F. Baskett (1997). “Analgesia and anaesthesia”. In David Skinner; Andrew Swain; Rodney Peyton; Colin Robertson (eds.). Cambridge Textbook of Accident and Emergency Medicine. Project co-ordinator, Fiona Whinster. Cambridge, UK: Cambridge University Press. p. 194. ISBN 9780521433792. Archived from the original on 2017-09-08.
- ^ Scriabine, Alexander (1999). “Discovery and development of major drugs currently in use”. In Ralph Landau; Basil Achilladelis; Alexander Scriabine (eds.). Pharmaceutical Innovation: Revolutionizing Human Health. Philadelphia: Chemical Heritage Press. p. 211. ISBN 9780941901215. Archived from the original on 2017-09-08.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 22. ISBN 9781284057560.
- ^ “The Top 300 of 2021”. ClinCalc. Retrieved 18 February 2021.
- ^ “Lidocaine – Drug Usage Statistics”. ClinCalc. Retrieved 18 February 2021.
- ^ “Local anaesthetic drugs”.
- ^ Cepeda MS, Tzortzopoulou A, Thackrey M, Hudcova J, Arora Gandhi P, Schumann R (December 2010). Tzortzopoulou A (ed.). “Adjusting the pH of lidocaine for reducing pain on injection”. The Cochrane Database of Systematic Reviews (12): CD006581. doi:10.1002/14651858.CD006581.pub2. PMID 21154371.(Retracted, see doi:10.1002/14651858.cd006581.pub3)
- ^ Derry S, Wiffen PJ, Moore RA, Quinlan J (July 2014). Derry S (ed.). “Topical lidocaine for neuropathic pain in adults”. The Cochrane Database of Systematic Reviews. 7 (7): CD010958. doi:10.1002/14651858.CD010958.pub2. PMC 6540846. PMID 25058164.
- ^ Sinha S, Schreiner AJ, Biernaskie J, Nickerson D, Gabriel VA (November 2017). “Treating pain on skin graft donor sites: Review and clinical recommendations”. The Journal of Trauma and Acute Care Surgery. 83 (5): 954–964. doi:10.1097/TA.0000000000001615. PMID 28598907. S2CID 44520644.
- ^ “Lidocaine/prilocaine spray for premature ejaculation”. Drug and Therapeutics Bulletin. 55 (4): 45–48. April 2017. doi:10.1136/dtb.2017.4.0469. PMID 28408390. S2CID 19110955.
- ^ Kumar M, Chawla R, Goyal M (2015). “Topical anesthesia”. Journal of Anaesthesiology Clinical Pharmacology. 31 (4): 450–6. doi:10.4103/0970-9185.169049. PMC 4676230. PMID 26702198.
- ^ Martí-Carvajal AJ, Simancas-Racines D, Anand V, Bangdiwala S (August 2015). “Prophylactic lidocaine for myocardial infarction”. The Cochrane Database of Systematic Reviews. 8 (8): CD008553. doi:10.1002/14651858.CD008553.pub2. PMC 8454263. PMID 26295202.
- ^ Slaughter LA, Patel AD, Slaughter JL (March 2013). “Pharmacological treatment of neonatal seizures: a systematic review”. Journal of Child Neurology. 28 (3): 351–64. doi:10.1177/0883073812470734. PMC 3805825. PMID 23318696.
- ^ Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, et al. (June 2018). “Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults”. The Cochrane Database of Systematic Reviews. 2018 (6): CD009642. doi:10.1002/14651858.cd009642.pub3. PMC 6513586. PMID 29864216.
- ^ Biller JA (2007). “Airway obstruction, bronchospasm, and cough”. In Berger AM, Shuster JL, Von Roenn JH (eds.). Principles and practice of palliative care and supportive oncology. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 297–307. ISBN 978-0-7817-9595-1.
Inhaled lidocaine is used to suppress cough during bronchoscopy. Animal studies and a few human studies suggest that lidocaine has an antitussive effect…
- ^ Birsa LM, Verity PG, Lee RF (May 2010). “Evaluation of the effects of various chemicals on discharge of and pain caused by jellyfish nematocysts”. Comp. Biochem. Physiol. C. 151 (4): 426–30. doi:10.1016/j.cbpc.2010.01.007. PMID 20116454.
- ^ Morabito R, Marino A, Dossena S, La Spada G (Jun 2014). “Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid”. Toxicon. 83: 52–8. doi:10.1016/j.toxicon.2014.03.002. PMID 24637105.
- ^ James G. Adams (2012). “32”. Emergency Medicine: Clinical Essentials. Elsevier Health Sciences. ISBN 9781455733941. Archived from the original on 2017-09-08.
- ^ Jackson D, Chen AH, Bennett CR (October 1994). “Identifying true lidocaine allergy”. J Am Dent Assoc. 125 (10): 1362–6. doi:10.14219/jada.archive.1994.0180. PMID 7844301.
- ^ Jump up to:a b Australian Medicines Handbook. Adelaide, S. Aust: Australian Medicines Handbook Pty Ltd. 2006. ISBN 978-0-9757919-2-9.[page needed]
- ^ Jump up to:a b Nielsen LJ, Lumholt P, Hölmich LR (October 2014). “[Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]”. Ugeskrift for Laeger. 176(44). PMID 25354008.
- ^ “Lidocaine”. Epocrates. Archived from the original on 2014-04-22.
- ^ Jump up to:a b “Lidocaine Hydrochloride and 5% Dextrose Injection”. Safety Labeling Changes. FDA Center for Drug Evaluation and Research (CDER). January 2014. Archived from the original on 2013-04-03.
- ^ “Lidocaine Viscous: Drug Safety Communication – Boxed Warning Required – Should Not Be Used to Treat Teething Pain”. FDA Center for Drug Evaluation and Research (CDER). June 2014. Archived from the original on 2014-07-14.
- ^ “Table 96–4. Drugs and Porphyria” (PDF). Merck Manual. Merck & Company, Inc. 2011. Archived from the original on 2014-04-20.
- ^ “Lidocaine – N01BB02”. Drug porphyrinogenicity monograph. The Norwegian Porphyria Centre and the Swedish Porphyria Centre. Archived from the original on 2014-04-20.
strong clinical evidence points to lidocaine as probably not porphyrinogenic
- ^ Khan, M. Gabriel (2007). Cardiac Drug Therapy (7th ed.). Totowa, NJ: Humana Press. ISBN 9781597452380.
- ^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man(8th ed.). Foster City, CA: Biomedical Publications. pp. 840–4. ISBN 978-0-9626523-7-0.
- ^ Picard J, Ward SC, Zumpe R, Meek T, Barlow J, Harrop-Griffiths W (February 2009). “Guidelines and the adoption of ‘lipid rescue’ therapy for local anaesthetic toxicity”. Anaesthesia. 64 (2): 122–5. doi:10.1111/j.1365-2044.2008.05816.x. PMID 19143686. S2CID 25581037.
- ^ Gulihar A, Robati S, Twaij H, Salih A, Taylor GJ (December 2015). “Articular cartilage and local anaesthetic: A systematic review of the current literature”. Journal of Orthopaedics. 12 (Suppl 2): S200-10. doi:10.1016/j.jor.2015.10.005. PMC 4796530. PMID 27047224.
- ^ Carterall, William A. (2001). “Molecular mechanisms of gating and drug block of sodium channels”. Sodium Channels and Neuronal Hyperexcitability. Novartis Foundation Symposia. 241. pp. 206–225. doi:10.1002/0470846682.ch14. ISBN 9780470846681.
- ^ Sheu SS, Lederer WJ (Oct 1985). “Lidocaine’s negative inotropic and antiarrhythmic actions. Dependence on shortening of action potential duration and reduction of intracellular sodium activity”. Circulation Research. 57 (4): 578–90. doi:10.1161/01.res.57.4.578. PMID 2412723.
- ^ Lewin NA, Nelson LH (2006). “Chapter 61: Antidysrhythmics”. In Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA, Nelson LH (eds.). Goldfrank’s Toxicologic Emergencies(8th ed.). New York: McGraw-Hill. pp. 963–4. ISBN 978-0-07-143763-9.
- ^ Thomson PD, Melmon KL, Richardson JA, Cohn K, Steinbrunn W, Cudihee R, Rowland M (April 1973). “Lidocaine pharmacokinetics in advanced heart failure, liver disease, and renal failure in humans”. Ann. Intern. Med. 78 (4): 499–508. doi:10.7326/0003-4819-78-4-499. PMID 4694036.
- ^ Collinsworth KA, Kalman SM, Harrison DC (1974). “The clinical pharmacology of lidocaine as an antiarrhythymic drug”. Circulation. 50 (6): 1217–30. doi:10.1161/01.CIR.50.6.1217. PMID 4609637.
- ^ Jump up to:a b Löfgren N (1948). Studies on local anesthetics: Xylocaine: a new synthetic drug (Inaugural dissertation). Stockholm, Sweden: Ivar Heggstroms. OCLC 646046738.[page needed]
- ^ Löfgren N, Lundqvist B (1946). “Studies on local anaesthetics II”. Svensk Kemisk Tidskrift. 58: 206–17.
- ^ Wildsmith JAW (2011). “Lidocaine: A more complex story than ‘simple’ chemistry suggests” (PDF). The Proceedings of the History of Anaesthesia Society. 43: 9–16. Archived (PDF) from the original on 2014-04-22.
- ^ “Lidocaine international forms and names”. Drugs.com. Retrieved 29 October 2017.
- ^ “Lidocaine Ingredient Summary”. Therapeutic Goods Administration. Retrieved 20 September 2018.
- ^ “Updating medicine ingredient names – list of affected ingredients”. Therapeutic Goods Administration. 24 June 2019. Retrieved 16 February 2020.
- ^ “The 2021 Prohibited List International Standard” (PDF). The World Anti-Doping Code. World Anti-Doping Agency (WADA). 1 January 2021. Archived from the original (PDF) on 13 May 2021. Retrieved 18 May 2021.
- ^ “New York Drug Threat Assessment”. National Drug Intelligence Center. November 2002. Archived from the original on 2012-08-12.
- ^ Pupka A, Sikora J, Mauricz J, Cios D, Płonek T (2009). “[The usage of synthol in the body building]”. Polimery W Medycynie. 39(1): 63–5. PMID 19580174.
- ^ Bernardo NP; Siqueira MEPB; De Paiva MJN; Maia PP (2003). “Caffeine and other adulterants in seizures of street cocaine in Brazil”. International Journal of Drug Policy. 14 (4): 331–4. doi:10.1016/S0955-3959(03)00083-5.
- ^ “UNITED STATES of America, Plaintiff-Appellee, v. Luis A. CUELLO, Alvaro Bastides-Benitez, John Doe, a/k/a Hugo Hurtado, and Alvaro Carvajal, Defendants-Appellants”. Docket No. 78-5314. United States Court of Appeals, Fifth Circuit. 1979-07-25. Archived from the original on 2012-05-24.
- ^ Winterman, Denise (2010-09-07). “How cutting drugs became big business”. BBC News Online. BBC News Magazine. Archivedfrom the original on 2 February 2017. Retrieved 20 January 2017.
- ^ “Revision Bulletin: Lidocaine and Prilocaine Cream–Revision to Related Compounds Test”. The United States Pharmacopeial Convention. November 30, 2007. Archived from the original on May 1, 2013.
- ^ Peterson, Michael E.; Talcott, Patricia A. (2013-08-07). Small Animal Toxicology. Elsevier Health Sciences. ISBN 978-0323241984. Archived from the original on 2017-09-08.
- ^ “FDA Freedom of Information Summary – Tributame” (PDF). Archived from the original (PDF) on 2015-05-18.
External links
- “Lidocaine”. Drug Information Portal. U.S. National Library of Medicine.
- “Lidocaine Transdermal Patch”. MedlinePlus.
- US patent 2441498, Nils Magnus Loefgren & Bengt Josef Lundqvist, “Alkyl glycinanilides”, published 1948-05-11, issued 1948-05-11, assigned to ASTRA APOTEKARNES KEM FAB
|
|
| CAS Number | |
|---|---|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.821 |
| Chemical and physical data | |
| Formula | C14H22N2O |
| Molar mass | 234.343 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 68 °C (154 °F) |
////////LIDOCAINE, lignocaine