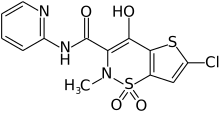
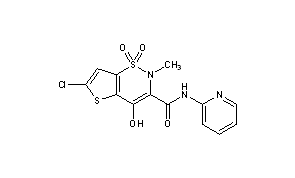
LORNOXICAM
chlortenoxicam
- Molecular FormulaC13H10ClN3O4S2
- Average mass371.819 Da
- Chlortenoxicam, Ro-13-9297
- ATC:M01AC05
- CCRIS 8589
- Ro 13-9297
Lorcam (Taisho Pharmaceutical Co.) / Xafon (Nycomed)

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 504-29-0 | C5H6N2 | 2-aminopyridine | 2-Pyridinamine |
| 7790-94-5 | ClHO3S | chlorosulfonic acid | Chlorosulfuric acid |
| 56946-84-0 | C5H5Cl2NO2S2 | 2,5-dichloro-N-methyl-3-thiophenesulfonamide | 3-Thiophenesulfonamide, 2,5-dichloro-N-methyl- |
| 3172-52-9 | C4H2Cl2S | 2,5-dichlorothiophene | Thiophene, 2,5-dichloro-
|

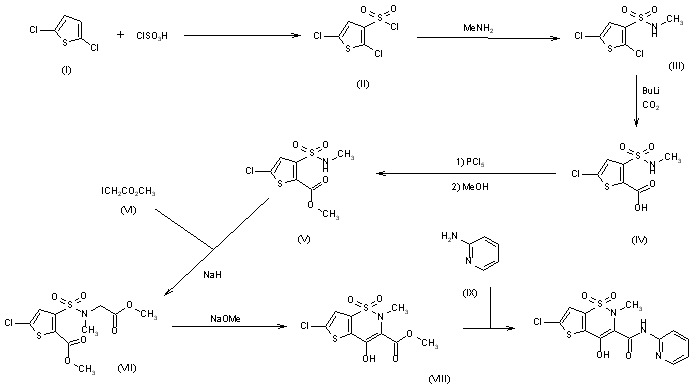
The sulfonation of 2,5-dichlorothiophene (I) with ClSO3H -SOCl2 gives 2,5-dichlorothiophene-3-sulfonic acid chloride (II), which by reaction with methylamine in CHCl3 yields the corresponding methylamide (III). The carboxylation of (III) with butyllithium and CO2 in ether affords 5-chloro-3-(N-methylsulfamoyl)thiophene-2-carboxylic acid (IV), which is esterified with PCl5 and methanol to the methyl ester (V). The condensation of (V) with methyl iodoacetate (VI) by means of NaH in DMF gives 5-chloro-3-[N-(methoxycarbonylmethyl)-N-methylsulfamoyl]thiophene-2-carboxylic acid methyl ester (VII), which is cyclized with sodium methoxide in methanol yielding 6-chloro-4-hydroxy-2-methyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic acid methyl ester 1,1-dioxide (VIII). Finally, this compound is treated with 2-aminopyridine (IX) in refluxing xylene.
Lornoxicam is an NSAID indicated in the treatment of mild to moderate pain, as well as rheumatoid arthritis and osteoarthritis.
Lornoxicam, also known as chlortenoxicam, is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic (pain relieving), anti-inflammatory and antipyretic (fever reducing) properties. It is available in oral and parenteral formulations.
It was patented in 1977 and approved for medical use in 1997.[1] Brand names include Xefo and Xefocam among others.
Lornoxicam (chlortenoxicam) is a new nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic, anti-inflammatory and antipyretic properties. Lornoxicam differs from other oxicam compounds in its potent inhibition of prostaglandin biosynthesis, a property that explains the particularly pronounced efficacy of the drug. Lornoxicam is approved for use in Japan.
Medical uses
Lornoxicam is used for the treatment of various types of pain, especially resulting from inflammatory diseases of the joints, osteoarthritis, surgery, sciatica, and other inflammations.[2]

Contraindications
The drug is contraindicated in patients who must not take other NSAIDs, possible reasons including salicylate sensitivity, gastrointestinal bleeding and bleeding disorders, and severe impairment of heart, liver or kidney function. Lornoxicam is not recommended during pregnancy and breastfeeding and is contraindicated during the last third of pregnancy.[2]
Adverse effects
Lornoxicam has side effects similar to other NSAIDs, most commonly mild ones like gastrointestinal disorders (nausea and diarrhea) and headache. Severe but seldom side effects include bleeding, bronchospasms and the extremely rare Stevens–Johnson syndrome.[2]
Interactions
Interactions with other drugs are typical of NSAIDs. Combination with vitamin K antagonists like warfarin increases the risk of bleeding. Combination with ciclosporin can lead to reduced kidney function, and to acute kidney injury in rare cases. Lornoxicam can also increase the adverse effects of lithium, methotrexate and digoxin and its derivatives. The effect of diuretics, ACE inhibitors and angiotensin II receptor antagonists can be reduced, but this is only relevant in patients with special risks like heart failure. As with piroxicam, cimetidine can increase plasma levels but is unlikely to cause relevant interactions.[3]
PAPER
https://www.mdpi.com/2218-0532/71/4/303
PATENT
CN 113480561
The present invention relates to the prepn. of high purity loroxicam. In particular, the prepn. method comprises a step of taking 6-chloro-4-hydroxy-2-methyl-2H-thieno[2,3-e]-1,2-Me thiazinecarboxylate-1,1-dioxide and 2-amino pyridine is used as the raw material and xylene is used as the solvent undergoes distn. reaction with solid acid catalyst, mixed gas obtained by the distn. reaction is condensed to obtain a condensate and solid acid catalyst is used to adsorb methanol in the condensate and the adsorbed condensate is recycled, filtering and refining to obtain loroxicam. The present inventive method distills out the methanol produced by the reaction to promote the pos. progress of the reaction and then catalyzes the absorption of methanol by H2SO4/MxOy solid super acid, so that the xylene returned to the reaction system does not contain methanol, which reduces the coking of the reaction, thereby improving product quality and yield. The prepd. lornoxicam has high purity, which can reach more than 99.9%, reduces the amt. of solvent and also suitable for industrial prodn.
PATENT
CN 112592356
The present invention relates to the prepn. of lornoxicam. In particular, the prepn. method comprises a step of taking 6-chloro-4-hydroxy-2-methyl-2-H-thieno[2,3-e]-1,2-thiazidecarboxylic acid Me ester-1,1-dioxide and 2-aminopyridine as raw materials, xylene is used as solvent, adding stabilizer, and carrying out aminolysis reaction, the solvent was removed by concn. under reduced pressure, adding org. solvent to make the slurry, filtering and refining to obtain lornoxicam. The inventive method uses p-toluene sulfonic acid as a stabilizer, while lowering the reaction temp., it promotes the reaction to proceed forward, and improve the product quality and yield; at the same time reduce the amt. of industrial solvents, the post-treatment process is optimized and the cost of the three wastes treatment is reduced.
PATENT
IN 2014CH02116
https://patentscope.wipo.int/search/en/detail.jsf?docId=IN211706269&_cid=P21-KV1YL4-08023-1
Example: 1Preparation of 6-chloro-4-hydroxy-l,l-dioxo-l,2-dihydro-lX6-thieno [2,3-e][l,2] thiazine-3-carboxylic acid methyl ester To the mixture of methanol ( 1000 ml) and 5-chloro-3-(methoxy carbonyl methyl sulfamoyl)-thiophene-2-carboxylicacid methyl ester ( 100 g ,0.305 moles), added sodium methoxide solution (200 ml ) at 25-30°C over a period of 30-45 min. The resulting mixture was stirred for 60 min at same temperature; allowed to heat at 65-75°C and stirred for 10-12 hrs. After completion of reaction, methanol was distilled out under reduced pressure to obtained titled residual product which is directly used to next step
(Example-2). Example: – 2:Preparation of 6-chloro-4-hydroxy-2-methyl-l,l-dioxo-l,2-dihydro-U6- thieno[2,3-e][l,2] thiazine-3-carboxylic acid methyl ester 6-chloro-4-hydroxy-1,1 -dioxo-1,2-dihydro-1 X,6-thieno [2,3-e][ 1,2] thiazine-3-carboxylic acid methyl ester was suspended in DM water (500 ml) and cooled to 10-15° C, dimethyl sulphate ( 70 g) was slowly added to the mixture at 10-15°C in 30 min. The reaction mixture was raised to 25-30°C and maintained for 2-3 hours at same temperature. After completion of reaction, mixture was cooled to 10-15°C, methylene dichloride (1600 ml) was added, reaction mixture pH was adjust to 1.0 -2.0 with hydrochloric acid at 10-15° C, stir reaction mixture to separate the layers. The methylene dichloride layer was distilled out completely at below 30°C to get an residue, followed by addition of methanol (60 ml) and distilled out methanol completely under vacuum at below 50°C to get an residue; further it was crystallized by addition of methanol 190 ml and stirred for 30 min at 50-55°C; cooled the reaction mixture at 25-30°C and stirred for 60 min at same temperature. The resultant solid was filtered, washed with methanol (40 ml) and dried at 50-55°C for 4 – 6 hrs to obtain the titled product
Example: 3Preparation of 6-Chloro-4-hydroxy-2-methyl-N-2-pyridinyl-2H-thieno[2,3-e]-l,2-thiazine-3-carboxamide 1,1-dioxide (Lornoxicam) 6-chloro-4-hydroxy-2-methyl-l, 1 -dioxo-1,2-dihydro-l X.6-thieno[2,3-e][l ,2] thiazine-3-carboxylic acid methyl ester ( 50 g 0.161 moles) was suspended in O-xylene (500 ml) and allow to stirred at 70-75°C to obtained clear solution. To this clear solution slowly added the mixture of THF ( 50 ml) solution of 2-Amino pyridine ( 14 g ) and ethyl magnesium bromide 2 molar solution (100 ml) at 70-75°C and allow to stirred for 3-4 hrs at same temperature. After completion of reaction, the dilute hydrochloric acid was added to the mixture at 10-15°C and stirred for 60 min. The resultant solid was filtered, washed with water (100 ml) to obtain crude Lornoxicam.
Example: 4Preparation of 6-Chloro-4-hydroxy-2-methyl-N-2-pyridinyl-2H-thieno[2,3-e)-l,2-thiazine-3-carboxamide 1,1-dioxide (Lornoxicam) 6-chloro-4-hydroxy-2-methyl-l,l-dioxo-l,2-dihydro-R6-thieno[2,3-e][l,2] thiazine-3-carboxylic acid methyl ester ( 50 g 0.161 moles) was suspended in O-xylene (500 ml) and allow to stirred at 70-75°C to obtained clear solution. To this clear solution slowly added the mixture of THF ( 50 ml) solution of 2-Amino pyridine ( 14 g ) and isopropyl magnesium bromide 2 molar solution (100 ml) at 70-75°C and allow to stirred for 3-4 hrs at same temperature. After completion of reaction, the dilute hydrochloric acid was added to the mixture at 10-15°C and stirred for 60 min. The resultant solid was filtered, washed with water (100 ml) to obtain crude Lornoxicam.
Example: 5Purification of Lornoxicam.The crude Lornoxicam was suspended in methanol (500 ml) and cooled to 5-10°C, resulting suspension was basified to pH 11-13 by using sodium hydroxide solution to get clear solution; followed by filtration through hyflo bed; the obtain filtrate was acidified to pH 4.5 – 5.0 with dil. HC1 (1:1) at 5-10°C; stirred the slurry for 30 min. at 5-10°C. The resultant solid was filtered, washed with DM water (100 ml) and dried at 50-55°C to obtained pure Lornoxicam.
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=IN211591584&_cid=P21-KV1YQ5-11871-1
.EXAMPLES:Preparation of Lornoxicam crudeExample ITo 1200ml o-xylene, 20gm Methyl-6-chloro-4-hydroxy-2-methyl-2//-thieno [2, 3-e] [1, 2] thiazine-3- carboxyate 1,1-dioxide and 6.44gm 2-aminopyridine was added. The reaction mass was stirred under nitrogen atmosphere. Temperature was raised to 140-145°C and maintained for 6hrs. The reaction mass was cooled to 30-35°C and nitrogen was removed. Reaction mass was further stirred for 3hrs- Filtered and washed twice with 50ml of o-xylene. 19.8gm of crude Lornoxicam was obtained. Purification of Lornoxicam crude
Example 219.8gm of crude Lornoxicam was added to the solvent mixture of water (5 vol with respect to Lornoxicam) and methanol (10 vol with respect to Lornoxicam) under stirring. Subsequently 48% sodium hydroxide was added to form a clear solution and 5% activated charcoal was further added. The reaction mass was heated to 50-55°C and stirred for around Ihr followed by filtration through Hyflo. To the filtrate, mixture of hydrochloric acid and water in the ratio of 1:1 was added at 50-55° C, til! the reaction mass reached pH of 2-3, and then stirred for around I hi*. The reaction mass was cooled to room temperature, filtered, and then washed with 1:1 mixture of methanol and water. Purified wet Lornoxicam was dried at 60-65°C for 6-8hrs. 19.1 gm of pure Lornoxicam was obtained. (HPLC purity- 99.95%)
Example 3!7.9gm of crude Lornoxicam (prepared as per example 1) was added to the solvent mixture of water (5 vol with respect to Lornoxicam) and methanol (10 vol with respect to Lornoxicam) under stirring. Subsequently 48% sodium hydroxide was added to form a clear solution, and 5% activated charcoal was further added. The reaction mass was heated to 50-55°C and stirred for around Ihr followed by filtration through Hyflo. To the filtrate, mixture of hydrochloric acid and water in the ratio of 1:1 was added at 50-55° C till the reaction mass reached pH of 2-3, and then stirred for around Ihr. The reaction mass was cooled to room temperature, filtered and then washed with 1:1 mixture of methanol and water. Purified wet Lornoxicam was dried at 60-65°C for 6-8hrs. 17.2 gm of pure Lornoxicam was obtained. (HPLC purity- 99.9%) clear solution and 5% activated charcoal was further added. The reaction mass was heated to 50-55°C and stirred for around lhr followed by filtration through Hyflo. To the filtrate, mixture of hydrochloric acid and water in the ratio of 1:1 was added at 50-55° C, till the reaction mass reached pH of 2-3, and then stirred for around lhr. The reaction mass was cooled to 30-35°C, filtered and then washed with 1:1 mixture of isopropyl alcohol and water. Purified wet Lornoxicam was dried at 60-65°C for 6-8hrs. 4.85 gm of pure Lornoxicam was obtained. (HPLC purity- 99.8%)
Example 55 gm of crude Lornoxicam (prepared as per example 1) was added to the solvent mixture of water (5 vol with respect to Lornoxicam) and ethanol (10 vol with respect to Lornoxicam) under stirring. Subsequently 48% sodium hydroxide was added to form a clear solution, and 5% activated charcoal was further added. The reaction mass was heated to 50-55°C and stirred for around lhr followed by filtration through Hyflo. To the filtrate, mixture of hydrochloric acid and water in the ratio of 1:1 was added at 50-55° C, til! the reaction mass reached pH of 2-3 and then stirred for around lhr. The reaction mass was cooled to 30-35°C and filtered, washed with 1:1 mixture of ethanol and water. Purified wet Lornoxicam was dried at 60-65°C for 6-8hrs. 4.8 gm of pure Lornoxicam was obtained. (HPLC purity- 99.8%)
Example 619.4 gm of crude Lornoxicam (prepared as per example I) was added to the solvent mixture of water (5 vol with respect to Lornoxicam) and methanol (10 vol with respect to Lornoxicam) under stirring. Subsequently 48% sodium hydroxide was added to form a clear solution, and 20% activated charcoal was further added. The reaction mass was stirred for around lhr at room temperature followed by filtration through Hyflo. To the filtrate, mixture of hydrochloric acid and water in the ratio of 1:1 was added till the reaction mass reached pH of 2-3 and then stirred for around 1 hr. The reaction mass was * filtered and washed with 1:1 mixture of methanol and water. Purified wet Lornoxicam was dried at 60-65°C for 6-8hrs. 18.9 gm of pure Lornoxicam was obtained. (HPLC purity- 99.3%).
PATENT
https://www.sciencedirect.com/science/article/abs/pii/S0968089603007624?via%
PATENT
https://patents.google.com/patent/WO2002000167A2/en
References
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 519. ISBN 9783527607495.
- ^ Jump up to:a b c Haberfeld H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. Xefo Filmtabletten. ISBN 978-3-85200-196-8.
- ^ Klopp T, ed. (2010). Arzneimittel-Interaktionen (in German) (2010/2011 ed.). Arbeitsgemeinschaft für Pharmazeutische Information. ISBN 978-3-85200-207-1.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Xefo, Xefocam others |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
By mouth, parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90–100% |
| Protein binding | 99% |
| Metabolism | CYP2C9 |
| Elimination half-life | 3–4 hours |
| Excretion | 2/3 liver, 1/3 kidney |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.646 |
| Chemical and physical data | |
| Formula | C13H10ClN3O4S2 |
| Molar mass | 371.81 g·mol−1 |
| 3D model (JSmol) | |
| |
|
| Pyrazolones / Pyrazolidines |
|
|---|---|
| Salicylates | |
| Acetic acid derivatives and related substances |
|
| Oxicams | |
| Propionic acid derivatives (profens) |
|
| N-Arylanthranilic acids (fenamates) |
|
| Coxibs | |
| Other | |
| Combinations | |
|
Items listed in bold indicate initially developed compounds of specific groups. #WHO-EM †Withdrawn drugs. ‡Veterinary use medications.
|
|
//////////LORNOXICAM, Ro-13-9297, TS-110, Anti-inflammatory, analgesic, chlortenoxicam, CCRIS 8589
CN1C(C(=O)NC2=CC=CC=N2)=C(O)C2=C(C=C(Cl)S2)S1(=O)=O
General References
- Balfour JA, Fitton A, Barradell LB: Lornoxicam. A review of its pharmacology and therapeutic potential in the management of painful and inflammatory conditions. Drugs. 1996 Apr;51(4):639-57. [Article]
- Vane JR: Introduction: mechanism of action of NSAIDs. Br J Rheumatol. 1996 Apr;35 Suppl 1:1-3. [Article]
- Radhofer-Welte S, Rabasseda X: Lornoxicam, a new potent NSAID with an improved tolerability profile. Drugs Today (Barc). 2000 Jan;36(1):55-76. [Article]
- Skjodt NM, Davies NM: Clinical pharmacokinetics of lornoxicam. A short half-life oxicam. Clin Pharmacokinet. 1998 Jun;34(6):421-8. [Article]
- Olkkola KT, Brunetto AV, Mattila MJ: Pharmacokinetics of oxicam nonsteroidal anti-inflammatory agents. Clin Pharmacokinet. 1994 Feb;26(2):107-20. [Article]
- Hitzenberger G, Radhofer-Welte S, Takacs F, Rosenow D: Pharmacokinetics of lornoxicam in man. Postgrad Med J. 1990;66 Suppl 4:S22-7. [Article]
- Pruss TP, Stroissnig H, Radhofer-Welte S, Wendtlandt W, Mehdi N, Takacs F, Fellier H: Overview of the pharmacological properties, pharmacokinetics and animal safety assessment of lornoxicam. Postgrad Med J. 1990;66 Suppl 4:S18-21. [Article]
- Bonnabry P, Leemann T, Dayer P: Role of human liver microsomal CYP2C9 in the biotransformation of lornoxicam. Eur J Clin Pharmacol. 1996;49(4):305-8. [Article]