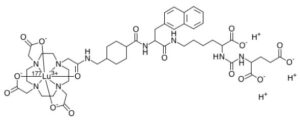
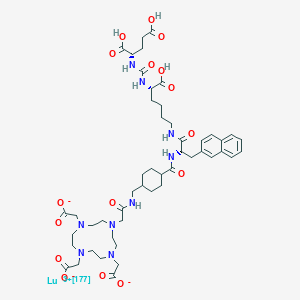
Lutetium Lu 177 vipivotide tetraxetan
FDA APPROVED 2022/3/23, Pluvicto
To treat prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer following other therapies
| Formula |
C49H65N9O16. Lu. 3H
|
|---|---|
| CAS |
1703749-62-5
|
| Mol weight |
1214.0819
|
|
Antineoplastic, Radioactive agent
|
|
| Disease |
Prostate cancer (PSMA positive)
|
|---|
ルテチウム(177Lu)ビピボチドテトラキセタン;
UNII-G6UF363ECX, WHO 11429
G6UF363ECX
177Lu-Psma-617
Vipivotide tetraxetan Lu-177
177Lu-Labeled PSMA-617
2-[4-[2-[[4-[[(2S)-1-[[(5S)-5-carboxy-5-[[(1S)-1,3-dicarboxypropyl]carbamoylamino]pentyl]amino]-3-naphthalen-2-yl-1-oxopropan-2-yl]carbamoyl]cyclohexyl]methylamino]-2-oxoethyl]-7,10-bis(carboxylatomethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetate;lutetium-177(3+)
Lutetium Lu 177 Vipivotide Tetraxetan is a radioconjugate composed of PSMA-617, a human prostate-specific membrane antigen (PSMA)-targeting ligand, conjugated to the beta-emitting radioisotope lutetium Lu 177 (177Lu), with potential antineoplastic activity against PSMA-expressing tumor cells. Upon intravenous administration of lutetium Lu 177 vipivotide tetraxetan, vipivotide tetraxetan targets and binds to PSMA-expressing tumor cells. Upon binding, PSMA-expressing tumor cells are destroyed by 177Lu through the specific delivery of beta particle radiation. PSMA, a tumor-associated antigen and type II transmembrane protein, is expressed on the membrane of prostatic epithelial cells and overexpressed on prostate tumor cells.
Lutetium (177Lu) vipivotide tetraxetan, sold under the brand name Pluvicto, is a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).[2] Lutetium (177Lu) vipivotide tetraxetan is a targeted radioligand therapy.[2][3]
The most common adverse reactions include fatigue, dry mouth, nausea, anemia, decreased appetite, and constipation.[2]
Lutetium (177Lu) vipivotide tetraxetan is a radioconjugate composed of PSMA-617, a human prostate-specific membrane antigen (PSMA)-targeting ligand, conjugated to the beta-emitting radioisotope lutetium Lu 177 (177Lu), with potential antineoplastic activity against PSMA-expressing tumor cells.[4] Upon intravenous administration of lutetium Lu 177 vipivotide tetraxetan, vipivotide tetraxetan targets and binds to PSMA-expressing tumor cells.[4] Upon binding, PSMA-expressing tumor cells are destroyed by 177Lu through the specific delivery of beta particle radiation.[4] PSMA, a tumor-associated antigen and type II transmembrane protein, is expressed on the membrane of prostatic epithelial cells and overexpressed on prostate tumor cells.[4]
Lutetium (177Lu) vipivotide tetraxetan was approved for medical use in the United States in March 2022.[2][5]
///////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
History[edit]
Efficacy was evaluated in VISION (NCT03511664), a randomized (2:1), multicenter, open-label trial that evaluated lutetium (177Lu) vipivotide tetraxetan plus best standard of care (BSoC) (n=551) or BSoC alone (n=280) in men with progressive, prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).[2] All participants received a GnRH analog or had prior bilateral orchiectomy.[2] Participants were required to have received at least one androgen receptor pathway inhibitor, and 1 or 2 prior taxane-based chemotherapy regimens.[2] Participants received lutetium (177Lu) vipivotide tetraxetan 7.4 GBq (200 mCi) every 6 weeks for up to a total of 6 doses plus BSoC or BSoC alone.[2]
The U.S. Food and Drug Administration granted the application for lutetium (177lu) vipivotide tetraxetan priority review and breakthrough therapy designations.[2]
References
- ^ “Highlights of prescribing information: PLUVICTOTM (lutetium Lu 177 vipivotide tetraxetan) injection, for intravenous use” (PDF). Advanced Accelerator Applications USA, Inc. Novartis. March 2022.
- ^ Jump up to:a b c d e f g h i j “FDA approves Pluvicto for metastatic castration-resistant prostate can”. U.S. Food and Drug Administration. 23 March 2022. Retrieved 23 March 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Neels OC, Kopka K, Liolios C, Afshar-Oromieh A (December 2021). “Radiolabeled PSMA Inhibitors”. Cancers. 13 (24): 6255. doi:10.3390/cancers13246255. PMC 8699044. PMID 34944875.
- ^ Jump up to:a b c d “Lutetium Lu 177 Vipivotide Tetraxetan (Code C148145)”. NCI Thesaurus. 28 February 2022. Retrieved 23 March 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “Novartis Pluvicto approved by FDA as first targeted radioligand therapy for treatment of progressive, PSMA positive metastatic castration-resistant prostate cancer” (Press release). Novartis. 23 March 2022. Retrieved 23 March 2022.
External links
- “Lutetium lu 177 vipivotide tetraxetan”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
| Clinical data | |
|---|---|
| Trade names | Pluvicto |
| Other names | 177Lu-PSMA-617, Lutetium Lu 177 vipivotide tetraxetan (USAN US) |
| License data | |
| Routes of administration |
Intravenous |
| Drug class | Radiopharmaceutical |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| 3D model (JSmol) | |
////////////Lutetium Lu 177 vipivotide tetraxetan, ルテチウム(177Lu)ビピボチドテトラキセタン, FDA 2022, APPROVALS 2022, PROSTRATE CANCER, WHO 11429
C1CC(CCC1CNC(=O)CN2CCN(CCN(CCN(CC2)CC(=O)[O-])CC(=O)[O-])CC(=O)[O-])C(=O)NC(CC3=CC4=CC=CC=C4C=C3)C(=O)NCCCCC(C(=O)O)NC(=O)NC(CCC(=O)O)C(=O)O.[Lu+3]
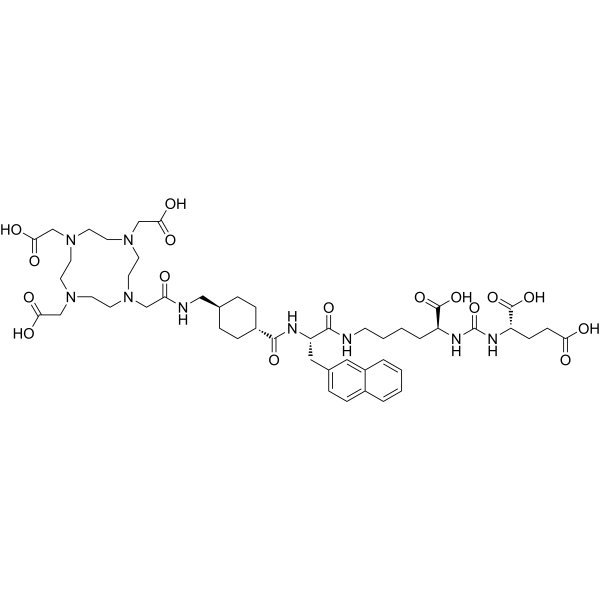
Vipivotide tetraxetan (Synonyms: PSMA-617)
CAS No. : 1702967-37-0
Vipivotide tetraxetan (PSMA-617) is a high potent prostate-specific membrane antigen (PSMA) inhibitor, with a Ki of 0.37 nM.