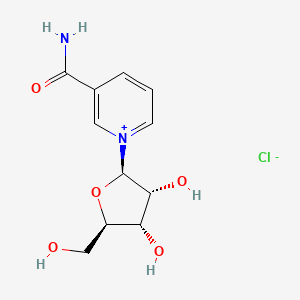
Nicotinamide riboside chloride
CAS 23111-00-4 CHLORIDE
CAS : 1341-23-7 (cation) 23111-00-4 (chloride) 445489-49-6 (Triflate)
3-Carbamoyl-1-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyridin-1-ium chloride
Nicotinamide ribose chloride
UNII-8XM2XT8VWI
MW 290.7 g/mol
1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium-3-carboxamide;chloride
C1=CC(=C[N+](=C1)C2C(C(C(O2)CO)O)O)C(=O)N.[Cl-]
Nicotinamide riboside; SRT647; SRT-647; SRT 647; Nicotinamide Riboside Triflate, α/β mixture
EH-301, nicotinamide riboside chloride,AND pterostilbene,, BY Elysium Health Inc
Nicotinamide riboside, also known as NR and SRT647, is a pyridine-nucleoside form of vitamin B3 that functions as a precursor to nicotinamide adenine dinucleotide or NAD+. NR blocks degeneration of surgically severed dorsal root ganglion neurons ex vivo and protects against noise-induced hearing loss in living mice. Nicotinamide riboside prevents muscle, neural and melanocyte stem cell senescence. Increased muscular regeneration in mice has been observed after treatment with nicotinamide riboside, leading to speculation that it might improve regeneration of organs such as the liver, kidney, and heart. Nicotinamide riboside also lowers blood glucose and fatty liver in prediabetic and type 2 diabetic models while preventing the development of diabetic peripheral neuropathy. Note: Nicotinamide Riboside chloride is a α/β mixture
Nicotinamide riboside (NR) is a pyridine–nucleoside form of vitamin B3 that functions as a precursor to nicotinamide adenine dinucleotide or NAD+.[1][2]
Chemistry
While the molecular weight of nicotinamide riboside is 255.25 g/mol,[3] that of its chloride salt is 290.70 g/mol.[4][5] As such, 100 mg of nicotinamide riboside chloride provides 88 mg of nicotinamide riboside.
History
Nicotinamide riboside (NR) was first described in 1944 as a growth factor, termed Factor V, for Haemophilus influenza, a bacterium that lives in and depends on blood. Factor V, purified from blood, was shown to exist in three forms: NAD+, NMN and NR. NR was the compound that led to the most rapid growth of this bacterium.[6] Notably, H. influenza cannot grow on nicotinic acid, nicotinamide, tryptophan or aspartic acid, which were the previously known precursors of NAD+.[7]
In 2000, yeast Sir2 was shown to be an NAD+-dependent protein lysine deacetylase,[8] which led several research groups to probe yeast NAD+ metabolism for genes and enzymes that might regulate lifespan. Biosynthesis of NAD+ in yeast was thought to flow exclusively through NAMN (nicotinic acid mononucleotide).[9][10][11][12][13]
When NAD+ synthase (glutamine-hydrolysing) was deleted from yeast cells, NR permitted yeast cells to grow. Thus, these Dartmouth College investigators proceeded to clone yeast and human nicotinamide riboside kinases and demonstrate the conversion of NR to NMN by nicotinamide riboside kinases in vitro and in vivo. They also demonstrated that NR is a natural product found in cow’s milk.[14][15]
Properties
Although it is a form of vitamin B3, NR exhibits unique properties that distinguish it from the other B3 vitamins—niacin and nicotinamide. In a head-to-head experiment conducted on mice, each of these vitamins exhibited unique effects on the hepatic NAD+ metabolome with unique kinetics, and with NR as the form of B3 that produced the greatest increase in NAD+ at a single timepoint.[16]
Different biosynthetic pathways are responsible for converting the different B3 vitamins into NAD+. The enzyme nicotinamide phosphoribosyltransferase (Nampt) catalyzes the rate-limiting step of the two-step pathway converting nicotinamide to NAD+. Two nicotinamide riboside kinases (NRK1 and NRK2) convert NR to NAD+ via a pathway that does not require Nampt.[14]
Animal studies have demonstrated that these enzymes respond differently to age and stress. In a mouse model of dilated cardiomyopathy, NRK2 mRNA expression increased, while Nampt mRNA expression decreased.[17] A similar increase in NRK1 and NRK2 expression has been observed in injured central and peripheral neurons.[18][19][20][21][22]
Niacin is known for its tendency to cause an uncomfortable flushing of the skin. This flushing is triggered by the activation of the GPR109A G-protein coupled receptor. NR does not activate this receptor,[23] and has not been shown to cause flushing in humans—even at doses as high as 2,000 mg/day.[16][24][25][26]
Despite being an NAD+ precursor, nicotinamide acts as an inhibitor of the NAD+-consuming sirtuin enzymes.[10] When sirtuins consume NAD+, they create nicotinamide and O-acetyl-ADP-ribose as products of the deacetylation reaction. Consistent with high-dose nicotinamide as a sirtuin inhibitor, NR and niacin, but not nicotinamide, have been shown to increase hepatic levels of O-acetyl-ADP-ribose.[16]
Commercialization
In 2004, Dartmouth Medical School researcher Dr. Charles Brenner discovered that NR could be converted to NAD+ via the eukaryotic nicotinamide riboside kinase biosynthetic pathway[14] Dartmouth was subsequently issued patents for nutritional and therapeutic uses of NR, in 2006.[27] ChromaDex licensed these patents in July 2012, and began to develop a commercially viable, full-scale process to bring NR to market.[28]
Human Clinical Testing
There have been five published clinical trials on groups of both men and women testing for safety. One of these trials studied NR in combination with pterostilbene,[29] while the other four examined the effects of NR alone.[16][24][25][26]
The first published clinical trial established the safety and characterized the pharmacokinetics of single doses of NR.[16] Since then, doses as high as 2,000 mg/day have been administered over periods as long as 12 weeks.[25] These studies show that NR can significantly increase levels of NAD+ and some of its associated metabolites in both whole blood and peripheral blood mononuclear cells.[16][24][26]
In a 12 week clinical trial of obese insulin-resistant men using 2000 mg/day, NR appeared safe, but did not improve insulin sensitivity or whole-body glucose metabolism.[26] In a trial of NR 250 mg plus 50 mg of pterostilbene, as well as with double this dose, the combined supplement raised NAD+ levels in a trial of older adults.[29]
PATENT
WO-2019126482
Crystalline form of nicotinamide riboside chloride, useful for treating motor neuron disease or ALS, infertility, kidney damage, and liver damage or fatty liver. Elysium Health in collaboration with Mayo Clinic , is developing EH-301 (clinical, in July 2019), a combination of nicotinamide riboside chloride and pterostilbene for the treatment of amyotrophic lateral sclerosis. See WO2019108878 , claiming use of composition comprising nicotinamide riboside and pterostilbene, for treating obesity.
Nicotinamide riboside is a pyridine-nucleoside form of niacin ( i.e ., vitamin B3) that serves as a precursor to nicotinamide adenine dinucleotide (NAD+). NAD+promotes cellular metabolism, mitochondrial function, and energy production. Currently, nicotinamide riboside is made through synthetic methods or fermentation processes. Because of its significant potential to confer health benefits when used as a dietary supplement, there exists a need to develop highly efficient and scalable processes for the manufacture and purification of nicotinamide riboside.
SUMMARY OF THE INVENTION
In certain aspects, the present invention provides a crystalline form of a compound having the structure of formula (I)
Example 1. Scale-Up Synthesis and Crystallization of Nicotinamide Riboside Chloride
900 kg of nicotinamide riboside triacetate and 2133 kg of methanol were charged to a reactor and mixed, then cooled to 0 °C. 747 kg of 7M mmmonia in methanol (i.e.,“methanolic NH3”) was slowly charged to the reactor at 0 °C. The reaction mixture was passed through a polish filter, then the reaction mixture was stirred for 14 hours. A sample from the reaction mixture was taken to assess reaction progress. Upon completion of the reaction, the reaction mixture was
placed under vacuum, then warmed to 20 °C to 25 °C for 4 hours. Vacuum was applied until solids formed. Once solids were formed, the resultant slurry was filtered on a Nutsche filter dryer. Solids were washed with 1422 kg of ethanol, then 1422 kg of acetone, then 1322 kg of methyl tert butyl ether (MTBE). The resultant solids were then dried at 40 °C. Product was formed with 60% yield. The process flow diagram for this reaction is shown in FIG. 6.
Example 2. Optional Secondary Isolation
The crystalline form may optionally undergo a second isolation process according to the following steps: The solids obtained in Example 1 were dissolved in purified water at 30 °C to 40 °C. Ethanol was slowly added to the solution and mixed for 10 hours, over which time the solids began to precipitate. MTBE was then added and mixed for 2 hours. The mixture was then filtered on a Buchner funnel, and the solids were washed with ethanol, then acetone, then MTBE. Solids were dried at 40 °C.
Example 3. Spectroscopic Data.
The crystalline form made by the process described in Examples 1 and 2 has an XRD spectrum substantially as shown in FIG. 1. The instrument utilized in collecting the XRD data is a Rigaku Smart Lab X-Ray diffraction system.
Specifically, in order to collect the XRD data, The Rigaku Smart-Lab X-ray diffraction system was configured for reflection Bragg-Brentano geometry using a line source X-ray beam. The X-ray source is a Cu Long Fine Focus tube that was operated at 40 kV and 44 mA. That source provides an incident beam profile at the sample that changes from a narrow line at high angles to a broad rectangle at low angles. Beam conditioning slits are used on the line X-ray source to ensure that the maximum beam size is less than 10 mm both along the line and normal to the line. The Bragg-Brentano geometry is a para-focusing geometry controlled by passive divergence and receiving slits with the sample itself acting as the focusing component for the optics. The inherent resolution of Bragg-Brentano geometry is governed in part by the diffractometer radius and the width of the receiving slit used. Typically, the Rigaku Smart-Lab is operated to give peak widths of 0.1 °2Q or less. The axial divergence of the X-ray beam is controlled by 5.0-degree Sober slits in both the incident and diffracted beam paths.
The samples were prepared in a low background Si holder using light manual pressure to keep the sample surface flat and level with the reference surface of the sample holder. The single crystal Si low background holder has a small circular recess (10 mm diameter and about 0.2 mm depth) that held between 20 and 25 mg of the sample. The samples were analyzed from 2 to 40
°2Q using a continuous scan of 6 °20 per minute with an effective step size of 0.02 °20. The data collection procedure used to analyze these samples was not validated. The peak lists were generated using PDXL2 v.2.3.1.0. The figures were created using PlotMon VI.00.
PATENT
WO2019108878 , claiming use of composition comprising nicotinamide riboside and pterostilbene, for treating obesity.
CLIP
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0186459
CLIP


References
- ^ Bogan, K.L., Brenner, C. (2008). “Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition”. Annu. Rev. Nutr. 28: 115–130. doi:10.1146/annurev.nutr.28.061807.155443. PMID 18429699.
- ^ Chi Y, Sauve AA (November 2013). “Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection”. Curr Opin Clin Nutr Metab Care. 16 (6): 657–61. doi:10.1097/MCO.0b013e32836510c0. PMID 24071780.
- ^ “Nicotinamide riboside”. pubchem.ncbi.nlm.nih.gov.
- ^ “GRAS Notices, GRN No. 635”. www.accessdata.fda.gov. Retrieved 18 February 2019.
- ^ “Spherix/ChromaDex GRAS submission” (PDF). FDA.gov. Retrieved 18 February2019.
- ^ Gingrich, W; Schlenk, F (June 1944). “Codehydrogenase I and Other Pyridinium Compounds as V-Factor for Hemophilus influenzae and H. parainfluenzae”. Journal of Bacteriology. 47 (6): 535–50. PMC 373952. PMID 16560803.
- ^ Belenky, P.; et al. (2007). “NAD+ Metabolism in Health and Disease”. Trends in Biochemical Sciences. 32 (1): 12–19. doi:10.1016/j.tibs.2006.11.006. PMID 17161604.
- ^ Imai, S.; et al. (2000). “Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase”. Nature. 403 (6771): 795–800. doi:10.1038/35001622. PMID 10693811.
- ^ Anderson; et al. (2003). “Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae”. Nature. 423 (6936): 181–185. doi:10.1038/nature01578. PMC 4802858. PMID 12736687.
- ^ Jump up to:a b Bitterman; et al. (2002). “Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast Sir2 and human SIRT1”. J. Biol. Chem. 277 (47): 45099–45107. doi:10.1074/jbc.m205670200. PMID 12297502.
- ^ Gallo; et al. (2004). “Nicotinamide clearance by pnc1 directly regulates sir2-mediated silencing and longevity”. Mol. Cell. Biol. 24 (3): 1301–1312. doi:10.1128/mcb.24.3.1301-1312.2004.
- ^ Panozzo, C.; et al. (2002). “Aerobic and anaerobic NAD+ metabolism in Saccharomyces cerevisiae”. FEBS Lett. 517 (1–3): 97–102. doi:10.1016/s0014-5793(02)02585-1. PMID 12062417.
- ^ Sandmeier, JJ; Celic, I; Boeke, JD; Smith, JS (March 2002). “Telomeric and rDNA silencing in Saccharomyces cerevisiae are dependent on a nuclear NAD(+) salvage pathway”. Genetics. 160 (3): 877–89. PMC 1462005. PMID 11901108.
- ^ Jump up to:a b c Bieganowki, P. & Brenner, C. (2004). “Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans”. Cell. 117 (4): 495–502. doi:10.1016/s0092-8674(04)00416-7. PMID 15137942.
- ^ Hautkooper, R.H.; et al. (2012). “Sirtuins as regulators of metabolism and healthspan”. Nat. Rev. Mol. Cell Biol. 13 (4): 225–238. doi:10.1038/nrm3293. PMC 4872805. PMID 22395773.
- ^ Jump up to:a b c d e f Trammell, Samuel A. J.; Schmidt, Mark S.; Weidemann, Benjamin J.; Redpath, Philip; Jaksch, Frank; Dellinger, Ryan W.; Li, Zhonggang; Abel, E. Dale; Migaud, Marie E.; Brenner, Charles (10 October 2016). “Nicotinamide riboside is uniquely and orally bioavailable in mice and humans”. Nature Communications. 7 (1): 12948. doi:10.1038/ncomms12948. PMC 5062546. PMID 27721479.
- ^ Diguet, Nicolas; Trammell, Samuel A.J.; Tannous, Cynthia; Deloux, Robin; Piquereau, Jérôme; Mougenot, Nathalie; Gouge, Anne; Gressette, Mélanie; Manoury, Boris; Blanc, Jocelyne; Breton, Marie; Decaux, Jean-François; Lavery, Gareth G.; Baczkó, István; Zoll, Joffrey; Garnier, Anne; Li, Zhenlin; Brenner, Charles; Mericskay, Mathias (22 May 2018). “Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy”. Circulation. 137 (21): 2256–2273. doi:10.1161/CIRCULATIONAHA.116.026099. PMID 29217642.
- ^ Vaur, Pauline; Brugg, Bernard; Mericskay, Mathias; Li, Zhenlin; Schmidt, Mark S.; Vivien, Denis; Orset, Cyrille; Jacotot, Etienne; Brenner, Charles; Duplus, Eric (December 2017). “Nicotinamide riboside, a form of vitamin B , protects against excitotoxicity-induced axonal degeneration”. The FASEB Journal. 31 (12): 5440–5452. doi:10.1096/fj.201700221RR. PMID 28842432.
- ^ Sasaki, Y.; Araki, T.; Milbrandt, J. (16 August 2006). “Stimulation of Nicotinamide Adenine Dinucleotide Biosynthetic Pathways Delays Axonal Degeneration after Axotomy”. Journal of Neuroscience. 26 (33): 8484–8491. doi:10.1523/JNEUROSCI.2320-06.2006. PMID 16914673.
- ^ Frederick, David W.; Loro, Emanuele; Liu, Ling; Davila, Antonio; Chellappa, Karthikeyani; Silverman, Ian M.; Quinn, William J.; Gosai, Sager J.; Tichy, Elisia D.; Davis, James G.; Mourkioti, Foteini; Gregory, Brian D.; Dellinger, Ryan W.; Redpath, Philip; Migaud, Marie E.; Nakamaru-Ogiso, Eiko; Rabinowitz, Joshua D.; Khurana, Tejvir S.; Baur, Joseph A. (August 2016). “Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle”. Cell Metabolism. 24 (2): 269–282. doi:10.1016/j.cmet.2016.07.005. PMC 4985182. PMID 27508874.
- ^ Cantó, Carles; Jiang, Lake Q.; Deshmukh, Atul S.; Mataki, Chikage; Coste, Agnes; Lagouge, Marie; Zierath, Juleen R.; Auwerx, Johan (March 2010). “Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle”. Cell Metabolism. 11 (3): 213–219. doi:10.1016/j.cmet.2010.02.006. PMC 3616265. PMID 20197054.
- ^ Rappou, Elisabeth; Jukarainen, Sakari; Rinnankoski-Tuikka, Rita; Kaye, Sanna; Heinonen, Sini; Hakkarainen, Antti; Lundbom, Jesper; Lundbom, Nina; Saunavaara, Virva; Rissanen, Aila; Virtanen, Kirsi A.; Pirinen, Eija; Pietiläinen, Kirsi H. (March 2016). “Weight Loss Is Associated With Increased NAD /SIRT1 Expression But Reduced PARP Activity in White Adipose Tissue”. The Journal of Clinical Endocrinology & Metabolism. 101 (3): 1263–1273. doi:10.1210/jc.2015-3054. PMID 26760174.
- ^ Cantó, Carles; Houtkooper, Riekelt H.; Pirinen, Eija; Youn, Dou Y.; Oosterveer, Maaike H.; Cen, Yana; Fernandez-Marcos, Pablo J.; Yamamoto, Hiroyasu; Andreux, Pénélope A.; Cettour-Rose, Philippe; Gademann, Karl; Rinsch, Chris; Schoonjans, Kristina; Sauve, Anthony A.; Auwerx, Johan (June 2012). “The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity”. Cell Metabolism. 15 (6): 838–847. doi:10.1016/j.cmet.2012.04.022. PMC 3616313. PMID 22682224.
- ^ Jump up to:a b c Airhart, Sophia E.; Shireman, Laura M.; Risler, Linda J.; Anderson, Gail D.; Nagana Gowda, G. A.; Raftery, Daniel; Tian, Rong; Shen, Danny D.; O’Brien, Kevin D.; Sinclair, David A. (6 December 2017). “An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers”. PLOS ONE. 12 (12): e0186459. doi:10.1371/journal.pone.0186459. PMC 5718430. PMID 29211728.
- ^ Jump up to:a b c Dollerup, Ole L; Christensen, Britt; Svart, Mads; Schmidt, Mark S; Sulek, Karolina; Ringgaard, Steffen; Stødkilde-Jørgensen, Hans; Møller, Niels; Brenner, Charles; Treebak, Jonas T; Jessen, Niels (August 2018). “A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects”. The American Journal of Clinical Nutrition. 108 (2): 343–353. doi:10.1093/ajcn/nqy132. PMID 29992272.
- ^ Jump up to:a b c d Martens, Christopher R.; Denman, Blair A.; Mazzo, Melissa R.; Armstrong, Michael L.; Reisdorph, Nichole; McQueen, Matthew B.; Chonchol, Michel; Seals, Douglas R. (29 March 2018). “Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults”. Nature Communications. 9 (1): 1286. doi:10.1038/s41467-018-03421-7. PMC 5876407. PMID 29599478.
- ^ Brenner, Charles (20 April 2006). “Nicotinamide riboside kinase compositions and methods for using the same”. Google Patents. Dartmouth College. Retrieved 19 February2019.
- ^ “ChromaDex Licenses Exclusive Patent Rights for Nicotinamide Riboside (NR) Vitamin Technologies”. 2012-07-16. Retrieved 15 February 2019.
- ^ Jump up to:a b Dellinger, Ryan W.; Santos, Santiago Roel; Morris, Mark; Evans, Mal; Alminana, Dan; Guarente, Leonard; Marcotulli, Eric (24 November 2017). “Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study”. NPJ Aging and Mechanisms of Disease. 3 (1): 17. doi:10.1038/s41514-017-0016-9. PMC 5701244. PMID 29184669.
Further reading
- “Press Release: NIH researchers find potential target for reducing obesity-related inflammation”. National Institutes of Health (NIH). 16 November 2015.
- Stipp, David (March 11, 2015). “Guest Blog: Beyond Resveratrol: The Anti-Aging NAD Fad”. Scientific American Blog Network.
- Zhang, H; Ryu, D; Wu, Y; Gariani, K; Wang, X; Luan, P; D’Amico, D; Ropelle, ER; Lutolf, MP; Aebersold, R; Schoonjans, K; Menzies, KJ; Auwerx, J (17 June 2016). “NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice”. Science. 352 (6292): 1436–43. doi:10.1126/science.aaf2693. PMID 27127236.
- Dolopikou CF, Kourtzidis IA, Margaritelis NV, Vrabas IS, Koidou I, Kyparos A, Theodorou AA, Paschalis V, Nikolaidis MG. (2019 Feb 6). Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. doi:10.1007/s00394-019-01919-4.
ADDITIONAL INFORMATION
High dose nicotinic acid is used as an agent that elevates high-density lipoprotein cholesterol, lowers low-density lipoprotein cholesterol and lower free fatty acids through a mechanism that is not completely understood. It was suggested that nicotinamide riboside might possess such an activity by elevating NAD in the cells responsible for reverse cholesterol transport. The discovery that the Wallerian degeneration slow gene encodes a protein fusion with NMN adenylyltransferase 1 indicated that increased NAD+ precursor supplementation might oppose neurodegenerative processes.
ChromaDex acquired intellectual property on uses and synthesis of NR from Dartmouth College, Cornell University, and Washington University and began distributing NR as Niagen in 2013. In November 2015 ChromaDex received New Dietary Ingredient (NDI) status for Niagen from the U.S. Food and Drug Administration (FDA) and the FDA issued a generally recognized as safe (GRAS) No Objection Letter for Nicotinamide Riboside Chloride (NR) on August 3, 2016.
REFERENCES
1: Chi Y, Sauve AA. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care. 2013 Nov;16(6):657-61. doi: 10.1097/MCO.0b013e32836510c0. Review. PubMed PMID: 24071780.
2: Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115-30. doi: 10.1146/annurev.nutr.28.061807.155443. Review. PubMed PMID: 18429699.
3: Ghanta S, Grossmann RE, Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: chemical and metabolic logic of acetyl-lysine modifications. Crit Rev Biochem Mol Biol. 2013 Nov-Dec;48(6):561-74. doi: 10.3109/10409238.2013.838204. Review. PubMed PMID: 24050258; PubMed Central PMCID: PMC4113336.
4: Yang Y, Sauve AA. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016 Dec;1864(12):1787-1800. doi: 10.1016/j.bbapap.2016.06.014. Review. PubMed PMID: 27374990.
5: Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008 Mar;324(3):883-93. doi: 10.1124/jpet.107.120758. Review. PubMed PMID: 18165311.
6: Kato M, Lin SJ. Regulation of NAD+ metabolism, signaling and compartmentalization in the yeast Saccharomyces cerevisiae. DNA Repair (Amst). 2014 Nov;23:49-58. doi: 10.1016/j.dnarep.2014.07.009. Review. PubMed PMID: 25096760; PubMed Central PMCID: PMC4254062.
7: Gerlach G, Reidl J. NAD+ utilization in Pasteurellaceae: simplification of a complex pathway. J Bacteriol. 2006 Oct;188(19):6719-27. Review. PubMed PMID: 16980474; PubMed Central PMCID: PMC1595515.
8: Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clin Transl Med. 2016 Dec;5(1):25. doi: 10.1186/s40169-016-0104-7. Review. PubMed PMID: 27465020; PubMed Central PMCID: PMC4963347.
9: Handschin C. Caloric restriction and exercise “mimetics”: Ready for prime time? Pharmacol Res. 2016 Jan;103:158-66. doi: 10.1016/j.phrs.2015.11.009. Review. PubMed PMID: 26658171; PubMed Central PMCID: PMC4970791.
10: Ruggieri S, Orsomando G, Sorci L, Raffaelli N. Regulation of NAD biosynthetic enzymes modulates NAD-sensing processes to shape mammalian cell physiology under varying biological cues. Biochim Biophys Acta. 2015 Sep;1854(9):1138-49. doi: 10.1016/j.bbapap.2015.02.021. Review. PubMed PMID: 25770681.
11: Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014 Aug;24(8):464-71. doi: 10.1016/j.tcb.2014.04.002. Review. PubMed PMID: 24786309; PubMed Central PMCID: PMC4112140.
12: Jaehme M, Slotboom DJ. Structure, function, evolution, and application of bacterial Pnu-type vitamin transporters. Biol Chem. 2015 Sep;396(9-10):955-66. doi: 10.1515/hsz-2015-0113. Review. PubMed PMID: 26352203.
13: Magni G, Di Stefano M, Orsomando G, Raffaelli N, Ruggieri S. NAD(P) biosynthesis enzymes as potential targets for selective drug design. Curr Med Chem. 2009;16(11):1372-90. Review. PubMed PMID: 19355893.
14: Mendelsohn AR, Larrick JW. Partial reversal of skeletal muscle aging by restoration of normal NAD⁺ levels. Rejuvenation Res. 2014 Feb;17(1):62-9. doi: 10.1089/rej.2014.1546. Review. PubMed PMID: 24410488.
15: Penberthy WT. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Curr Drug Metab. 2007 Apr;8(3):245-66. Review. PubMed PMID: 17430113.
16: Gazzaniga F, Stebbins R, Chang SZ, McPeek MA, Brenner C. Microbial NAD metabolism: lessons from comparative genomics. Microbiol Mol Biol Rev. 2009 Sep;73(3):529-41, Table of Contents. doi: 10.1128/MMBR.00042-08. Review. PubMed PMID: 19721089; PubMed Central PMCID: PMC2738131.
17: Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004 Jan;61(1):19-34. Review. PubMed PMID: 14704851.
18: Magni G, Orsomando G, Raffelli N, Ruggieri S. Enzymology of mammalian NAD metabolism in health and disease. Front Biosci. 2008 May 1;13:6135-54. Review. PubMed PMID: 18508649.
19: Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007 Jan;32(1):12-9. Review. Erratum in: Trends Biochem Sci. 2008 Jan;33(1):1. PubMed PMID: 17161604.
20: Niven DF, O’Reilly T. Significance of V-factor dependency in the taxonomy of Haemophilus species and related organisms. Int J Syst Bacteriol. 1990 Jan;40(1):1-4. Review. PubMed PMID: 2145965.
 |
|
 |
|
| Names | |
|---|---|
| Other names
1-(β-D-Ribofuranosyl)nicotinamide; N-Ribosylnicotinamide
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
|
PubChem CID
|
|
| Properties | |
| C11H15N2O5+ | |
| Molar mass | 255.25 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
///////////// EH-301, EH 301, EH301, Nicotinamide riboside, SRT647, SRT-647, SRT 647, Nicotinamide Riboside Triflate, α/β mixture
C1=CC(=C[N+](=C1)C2C(C(C(O2)CO)O)O)C(=O)N.[Cl-]