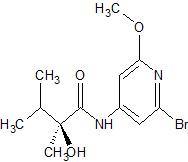
(S)-N-(2-bromo-6-methoxypyridin-4-yl)-2-hydroxy-2,4-dimethylpentanamide
(S)-N-(2-Bromo-6-methoxypyridin-4-yl)-2-hydroxy-2,4-dimethylpentanamide
CAS 1433905-44-2

Nonsteroidal antiandrogens
HPLC (Daicel Chiralpak IC 250 × 4.6 mm, 5 μm, n-heptane/IPA/TFA 930:70:1, 1 mL·min–1, 25 °C, UV 210 nm): tr (minor) = 5.1 min, tr (major) = 5.9 min.
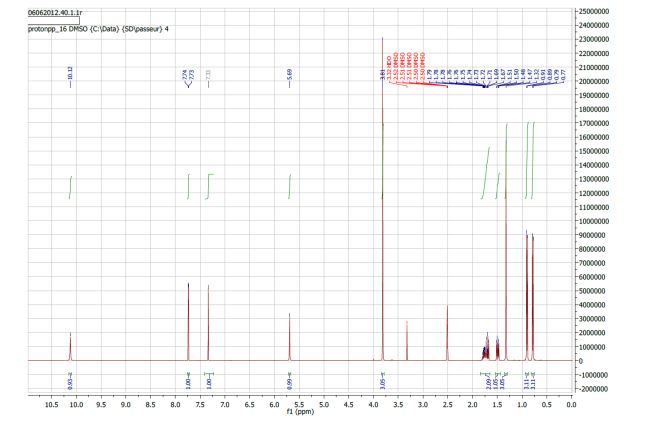
NMR 1H (400 MHz, DMSO-d6): 10.0 (sl, 1H); 7.73 (s, 1H); 7.33 (s, 1H); 5.70 (sl, 1H); 3.80 (s, 3H); 1.79–1.67 (m, 2H); 1.49 (dd, J = 13.6 and 5.2 Hz, 1H); 1.32 (s, 3H); 0.89 (d, J = 6.4 Hz, 3H); 0.78 (d, J = 6.4 Hz, 3H).
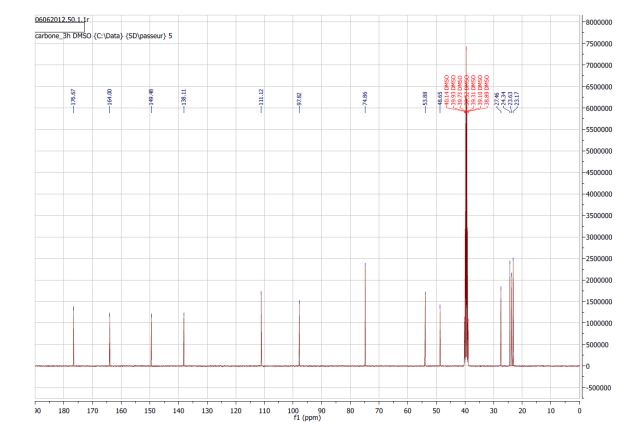
NMR 13C (100 MHz, DMSO-d6): 176.7, 164.0, 149.5, 138.1, 111.1, 74.9, 53.9, 48.6, 27.5, 24.3, 23.6, 23.2.
ESI-HRMS(m/z) calcd for C13H20BrN2O3+ [M+H]+ 331.0652 found 331.0654.
PATENT
Synthesis 71
(R)-2-Hydroxy-2,4-dimethyl-pentanoic acid (2-bromo-6-methoxy-pyridin-4-yl)-amide
(Compound 71A)
(S)-2-Hydroxy-2,4-dimethyl-pentanoic acid (2-bromo-6-methoxy-pyridin-4-yl)-amide
(Compound 71 B)
The two enantiomers of the racemic mixture prepared in Synthesis 41 were separated by HPLC (high pressure liquid chromatography) on a chiral stationary phase Chiralpak type la, Chiral Technologies, diameter 2 cm, length 25 cm, eluting with 93/7 (v/v) heptane / isopropanol containing 0.1 % (v/v) trifluoroacetic acid. The flow rate was 18 mL/minute. The injection volume was 1 mL of a solution of 20 mg of the racemic mixture dissolved in a 1 /1 (v/v) mixture of heptane / isopropanol. The retention times of the two enantiomers were 8.38 minutes and 9.70 minutes. After 6 injections, 40 mg of the two enantiomers were obtained as oils after solvent evaporation.
Analysis 71
Further analysis was performed using chiral HPLC (Chiralpak type la, Chiral
Technologies, 250 x 4, 6 mm, eluent 93/7 (v/v) heptane / isopropanol containing 0.1 % (v/v) trifluoroacetic acid with a flow rate of 1 mL/minute for 20 minutes. Compound 71 A had a retention time of 6.77 minutes, and Compound 71 B had a retention time of 8.71 minutes.
The absolute configuration of Compound 71 B was determined using X-ray diffraction (XRD), and found to be the (S) configuration. Accordingly, Compound 71 A was determined to be in the (R) configuration.

a(a) H2O2, TFA, 80%. (b) H2SO4, HNO3, 73%. (c) Fe, NH4Cl, EtOH, 51%. (d) MeOH, NaOH, MW 120 °C, 7 bar, 84%. (e) DCC, pyruvic acid, NMP, 29%. (f) i-BuMgCl, THF, 34%. (g) Chiral HPLC separation, 45%.
PAPER
http://pubs.acs.org/doi/abs/10.1021/acs.oprd.6b00392
Process Development and Crystallization in Oiling-Out System of a Novel Topical Antiandrogen
ACS Editors’ Choice – This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.

An efficient route to (S)-N-(2-bromo-6-methoxypyridin-4-yl)-2-hydroxy-2,4-dimethylpentanamide 1, a new topical antiandrogen, is described. The target compound has been manufactured on kilogram scale with an overall yield of 25% (HPLC purity 98.8% and >99% ee) from citrazinic acid. The key amide coupling between aminopyridine 4 and α-hydroxy-acid 6 was performed using a temporary protecting group to facilitate the acyl chloride formation. Aminopyridine 4 was manufactured from commercially available citrazinic acid via dibromide formation using phosphorus(V) oxybromide followed by mono SNAr reaction with sodium methoxide and a final Hofmann rearrangement. Enantiopure α-hydroxy-acid 6 was obtained using an enantioselective cyanosilylation followed by salt resolution with (S)-α-methyl benzylamine. The absolute configuration of compound 1 was determined with anomalous scattering and the final crystallization of API was performed after seeding a liquid–liquid mixture below the monotectic temperature and afforded a crystalline powder presenting a “desert rose” shape clusters.
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
///////Nonsteroidal antiandrogens,
Brc1cc(NC(=O)[C@@](C)(O)C(C)C)cc(OC)n1