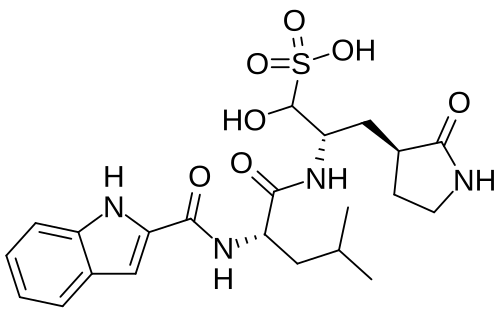
Olgotrelvir
STI-1558, HY-156655, CS-0887294, STI 1558, HY 156655, CS 0887294
Cas 2763596-71-8
494.6 g/mol, C22H30N4O7S, ZP3BDH359D
C22H30N4O7S 3-Pyrrolidinepropaney, α-hydroxy-β-[[(2S)-2-[(1H-indol-2-ylcarbonyl)amino]-4-methyl-1-oxopentyl]amino]-2-oxo-, (βS,3S)-
- (2S)-2-[(S)-2-(1H-Indole-2-carboxamido)-4-methylpentanamido]-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl]propane-1-sulfonic acid
- 3-Pyrrolidinepropanesulfonic acid, alpha-hydroxy-beta-[[(2S)-2-[(1H-indol-2-ylcarbonyl)amino]-4-methyl-1-oxopentyl]amino]-2-oxo-, (betaS,3S)-
Olgotrelvir sodium, C22H30N4O7S.Na, CAS 2763596-71-8
3-Pyrrolidinepropanesulfonic acid, α-hydroxy-β-[[(2S)-2-[(1H-indol-2-ylcarbonyl)amino]-4-methyl-1-oxopentyl]amino]-2-oxo-, sodium salt (1:1), (βS,3S)-
Olgotrelvir (STI-1558) is an experimental antiviral medication being studied as a potential treatment for COVID-19. It is believed to work by inhibiting the SARS-CoV-2 main protease (Mpro), a key enzyme that SARS-CoV-2 needs to replicate,[1][2][3][4] and by blocking viral entry.[2][5]
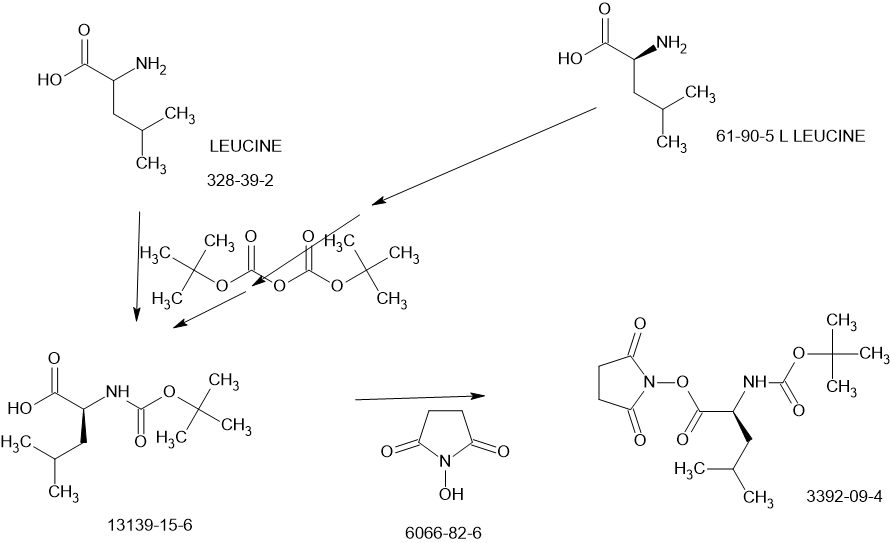
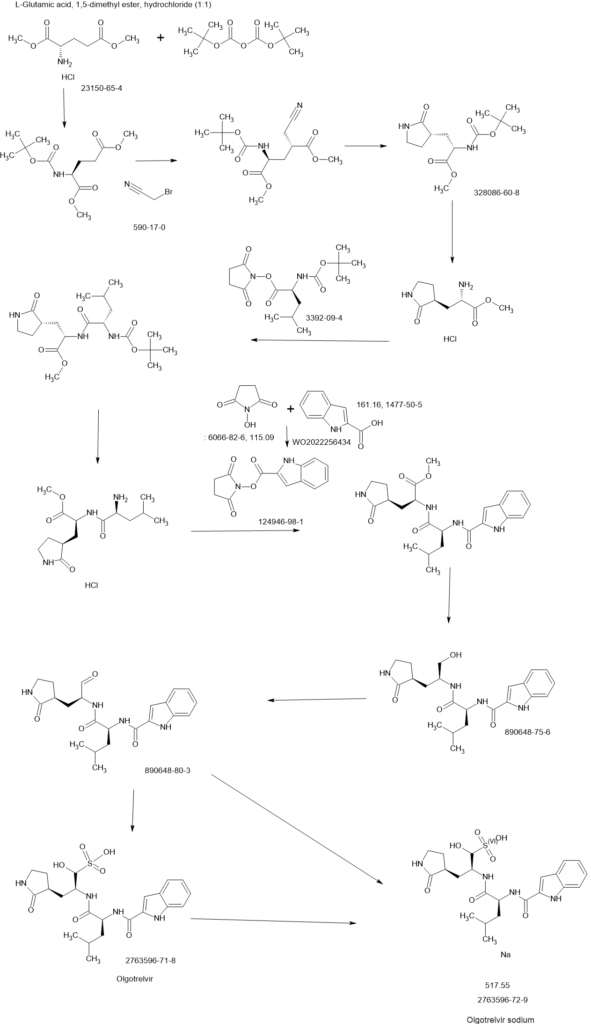
SCHEME
Main
PATENT
US20230322668 – PROTEASE INHIBITORS AS ANTIVIRALS
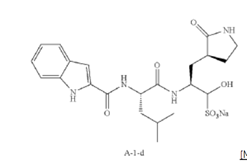
Example S1: Synthesis of Compounds A-1-a, A-1-b, A-1-c and A-1-d
US20230026438 – PROTEASE INHIBITORS AS ANTIVIRALS
WO2022256434 – PROTEASE INHIBITORS AS ANTIVIRALS
Example S1: Synthesis of Compounds A-1-a, A-1-b, A-1-c and A-1-d
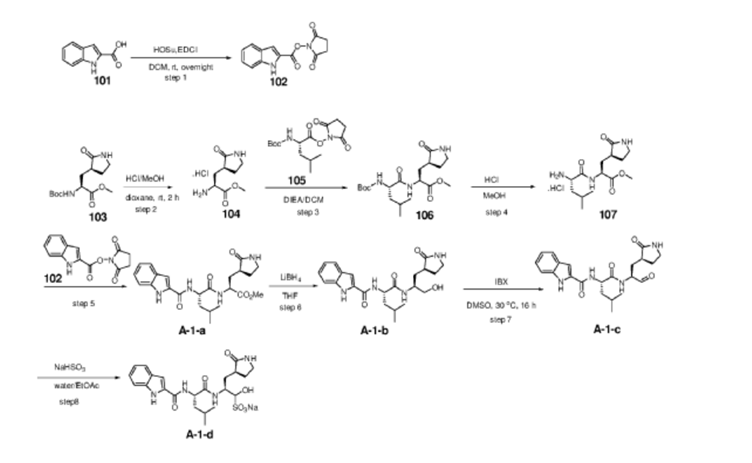
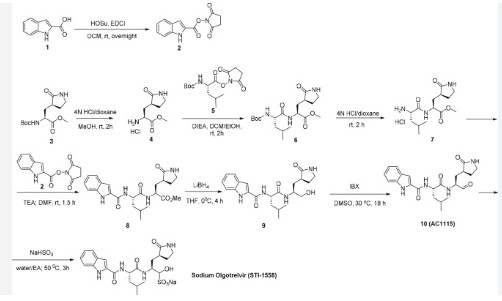
[00224] To a dichloromethane (2.5 L) solution of 1H-indole-2-carboxylic acid (compound 101) (200 g, 1.24 mol) and N-hydroxy succinimide (157.1 g, 1.37 mol) was added EDCI (286 g, 1.49 mmol) at 0℃. After stirring at room temperature overnight, the solvent was removed under reduced pressure. The resulting solid was triturated with deionized water, and the solid was collected and dried under reduced pressure to give the compound 102 as a light-brown solid (310 g, 96%).
1H NMR (400 MHz, CDCl3) δ 9.01 (s, 1H), 7.70 (d, J = 8.2 Hz, 1H), 7.49 – 7.35 (m, 3H), 7.19 (t, J = 7.4 Hz, 1H), 2.92 (s, 4H).
[00225] To a stirred mixture of methyl (2S)-2-{[(tert-butoxy)carbonyl]amino}-3-[(3S)-2- oxopyrrolidin-3-yl]-propanoate (compound 103) (500 g, 1748.24 mmol) in MeOH (200 mL) was
added 4M HCl in 1,4-dioxane (2000 mL) at room temperature. The mixture was stirred at rt for 2 h. LCMS indicated completion of the reaction. The reaction mixture was concentrated under reduced pressure to afford methyl (2S)-2-amino-3-[(3S)-2-oxopyrrolidin-3-yl]propanoate hydrochloride salt (compound 104) (389 g, 1721 mmol, 98%) as a light-yellow solid, which was used for next step without further purification. LCMS= [M+H]+: 187.1.
[00226] To a stirred mixture of methyl (2S)-2-amino-3-[(3S)-2-oxopyrrolidin-3-yl]propanoate hydrochloride (389 g, 1721 mmol) (compound 104) and DIEA (866.162 mL, 5240.94 mmol) in DCM (1800 mL) and EtOH (500 mL) was added 2,5-dioxopyrrolidin-1-yl (2R)-2-{[(tert-butoxy)carbonyl]amino}-4-methyl-pentanoate (compound 105) (573.66 g, 1746.98 mmol) at room temperature. The reaction mixture was stirred at room temperature for 2 h. LCMS indicated completion of the reaction. The reaction mixture was successively washed with water (1.0 L x 2), 0.5 M HCl (1.1 L), sat. NaHCO3 (1 L) and water (1 L). The organic layer was separated, dried with anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford the compound 106 (700 g, 1752.23 mmol, >99%) as a light-yellow solid, which was used for next step without further purification. LCMS = [M+H]+: 400.3.
(400 MHz, DMSO-d6) δ 8.32 (d, J = 8.0 Hz, 1H), 7.62 (s, 1H), 6.88 (d, J = 8.0 Hz, 1H), 4.40 – 4.28 (m, 1H), 3.94 (dd, J = 15.1, 8.1 Hz, 1H), 3.74 – 3.52 (m, 3H), 3.15 (t, J = 8.8 Hz, 1H), 3.06 (dd, J = 16.4, 9.2 Hz, 1H), 2.33 (t, J = 9.2 Hz, 1H), 2.14 – 2.00 (m, 2H), 1.68 – 1.51 (m, 3H), 1.42 – 1.34 (m, 11H), 0.87 (dd, J = 11.4, 6.6 Hz, 6H).
[00227] A mixture of methyl (2S)-2-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-4-methylpentanamido]-3-[(3S)-2-oxopyrrolidin-3-yl]propanoate (compound 106) (590 g, 1476.88 mmol) in HCl/dioxane (3 L) was stirred at room temperature for 2 h. LC-MS indicated completion of the reaction. The reaction mixture was concentrated under reduced pressure to give compound 107 as a yellow solid (490 g, 99%), which was used for next step without further purification.
LCMS = [M+H]+: 300.2.
[00228] To a stirred mixture of methyl (S)-2-((S)-2-amino-4-methylpentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate hydrochloride (compound 107) (418 g, 1235 mmol) and TEA (519.020 mL, 3734.03 mmol) in DMF (2500 mL) at room temperature was added 2,5-dioxopyrrolidin-1-yl 1H-indole-2-carboxylate (compound 102) (353 g, 1369.15 mmol) . The reaction mixture was stirred for 1.5 h. LCMS indicated that the reaction was complete. EtOAc (6 L) was added into the reaction mixture, which was then washed with brine (6 L x 6). The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated down under reduced
pressure. Compound A-1-a was obtained as an off-white solid (414 g. Y: 76%), which was used for next step without further purification. LCMS = [M+H]+: 443.3. 1H NMR (400 MHz, DMSO-d6) δ 11.55 (s, 1H), 8.54 (t, J = 12.2 Hz, 1H), 8.40 (d, J = 8.1 Hz, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.43 (d, J = 8.2 Hz, 1H), 7.24 (t, J = 10.3 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 4.65 – 4.50 (m, 1H), 4.44 – 4.28 (m, 1H), 3.72 – 3.55 (s, 3H), 3.19 – 3.06 (m, 2H), 2.36 (ddd, J = 13.8, 10.3, 4.0 Hz, 1H), 2.16 – 2.03 (m, 2H), 1.79 – 1.49 (m, 5H), 0.92 (dt, J = 14.4, 7.2 Hz, 6H).
[00229] To a stirred solution of methyl (S)-2-((S)-2-(1H-indole-2-carboxamido)-4-methylpentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate (compound A-1-a) (500 g, 1131 mmol) in THF (20 L) LiBH4 (74 g, 3393 mmol) was added portionwise at 0 ℃. The reaction mixture was stirred at 0 ℃ for 4 h. After reaction was completed (monitored by LCMS), the reaction mixture was quenched with sat. aqueous NH4Cl until no more gas formed. The mixture was washed with brine (5 L x 4), organic layer was collected, dried over anhydrous sodium sulfate, filtered, and concentrated down in vacuum. The resulting residue was purified by silica column chromatography (DCM : MeOH = 15 : 1) to give the desired product compound A-1-b (310 g, 66%) as a white solid. LCMS = [M+H]+: 415.2.
NMR (400 MHz, DMSO-d6) δ 11.57 (s, 1H), 8.39 (d, J = 8.2 Hz, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 7.52 (s, 1H), 7.42 (d, J = 8.3 Hz, 1H), 7.26 (d, J = 1.4 Hz, 1H), 7.17 (t, J = 7.6 Hz, 1H), 7.03 (t, J = 7.5 Hz, 1H), 4.67 (t, J = 5.6 Hz, 1H), 4.50 (td, J = 9.7, 5.0 Hz, 1H), 3.80 (s, 1H), 3.40 – 3.28 (m, 1H), 3.28 – 3.20 (m, 1H), 3.15 – 2.99 (m, 2H), 2.33 – 2.20 (m, 1H), 2.12 (dt, J = 17.8, 9.4 Hz, 1H), 1.86 – 1.75 (m, 1H), 1.75 – 1.64 (m, 2H), 1.56 (ddd, J = 19.3, 9.6, 6.9 Hz, 2H), 1.45 – 1.35 (m, 1H), 0.91 (dd, J = 15.6, 6.3 Hz, 6H).
[00230] To a stirred solution of N-((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)-1H-indole-2-carboxamide (compound A-1-b) (8.3 g, 20 mmol) in DMSO (60 mL) was added 2-iodoxybenzoic acid (IBX) (11.2 g, 40 mmol) at room temperature. The reaction mixture was stirred at 30 ℃ for 18 h, and LCMS indicated completion of the reaction. The reaction mixture was diluted with EtOAc (300 mL) and filtered. The filtrate was washed with mixture of brine and sat. aqueous NaHCO3 (1:1 to 5:1, 200 mL x 5). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated down at rt to afford crude product. THF (40 mL) was added, and the mixture was stirred overnight at room temperature. The resulting solid was collected and dried under vacuum to yield the desired product N-((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)-propan-2-yl)amino)pentan-2-yl)-1H-indole-2-carboxamide (compound A-1-c) as a white solid (2.5 g, 31%). LCMS = [M+H]+:
413.2. 1H NMR (400 MHz, CDCl3) δ 9.75 (s, 1H), 9.49 (s, 1H), 8.64 (s, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.4 Hz, 1H), 7.27 (d, J = 8.4 Hz, 1H), 7.14-7.05 (m, 2H), 7.01 (s, 1H), 6.34 (s, 1H), 4.90 (s, 1H), 4.34 (s, 1H), 3.27–3.22 (m, 2H), 2.43 (s, 1H), 2.30 (s, 1H), 2.01-1.96 (m, 1H), 1.94-1.91 (m, 1H) 1.88 – 1.65 (m, 4H), 1.00-0.98 (m, 6H).
[00231] To a stirred solution of N-((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)-1H-indole-2-carboxamide (compound A-1-c) (31 g, 75.25 mmol) in EtOAc (300 mL) at room temperature was added a solution of NaHSO3 (27.56 mg, 72.73 mmol) in water (100 mL). The reaction mixture was heated at 50 ℃ for 3 h. After completion of reaction (monitored by LCMS), the organic layer was separated and removed. The aqueous layer was washed with EtOAc (100 mL x 5), concentrated down to remove remaining EtOAc, and then lyophilized to provide the desired product sodium (2S)-2-((S)-2-(1H-indole-2-carboxamido)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (compound A-1-d) as off-white solid (32 g, 85%). LCMS = [M-Na+2H]+: 495.2. 1H NMR (400 MHz, DMSO-d6) δ 11.57 (s, 1H), 8.45 (dd, J = 20.7, 8.2 Hz, 1H), 7.72 (dd, J = 48.9, 9.2 Hz, 1H), 7.62 (d, J = 8.1 Hz, 1H), 7.50 – 7.38 (m, 2H), 7.25 (dd, J = 5.1, 1.4 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 5.43 (dd, J = 50.7, 5.9 Hz, 1H), 4.57 – 4.41 (m, 1H), 4.33 – 4.03 (m, 1H), 4.01 – 3.82 (m, 1H), 3.19 – 2.92 (m, 2H), 2.29 – 2.08 (m, 2H), 2.06 – 1.90 (m, 1H), 1.83 – 1.51 (m, 5H), 1.00 – 0.83 (m, 6H).
PAPER
https://www.sciencedirect.com/science/article/pii/S2666634023004026
Mechanism of action
Olgotrelvir is a prodrug that first converts to its active form, AC1115.[2][5] AC1115 is believed to work by inhibiting the SARS-CoV-2 main protease (also known as 3C-like protease). This protein is a crucial enzyme responsible for cleaving viral polyproteins into functional subunits essential for viral replication. By binding to the active site of the protease, the drug prevents this cleavage process, effectively halting viral assembly and impeding the virus’s ability to produce future virions.[1][2][3][5]
Olgotrelvir also appears to inhibit cathepsin L (CTSL),[2][5] a protein implicated in facilitating viral entry of SARS-CoV-2 into the host cell.[2][5][6]
Clinical trials
In September 2023, the drug’s developer, Sorrento Therapeutics, announced top-line data that olgotrelvir had met its primary endpoints in a phase III clinical trial that enrolled 1,212 patients with mild or moderate COVID-19. The drug appeared to shorten the recovery time of 11 COVID-19 symptoms in olgotrelvir-treated patients by 2.4 days on average compared to patients in the placebo group. The drug was also shown to reduce the viral load at day 4 in treated patients compared to the placebo group. Side effects were mostly mild and infrequent, with the most common being nausea (1.5% vs. 0.2%) and skin rash (3.3% vs. 0.3%), which occurred more often in the olgotrelvir group.[7][8][9]
References
- ^ Jump up to:a b Tong X, Keung W, Arnold LD, Stevens LJ, Pruijssers AJ, Kook S, et al. (November 2023). “Evaluation of in vitro antiviral activity of SARS-CoV-2 Mpro inhibitor pomotrelvir and cross-resistance to nirmatrelvir resistance substitutions”. Antimicrobial Agents and Chemotherapy. 67 (11): e0084023. doi:10.1128/aac.00840-23. PMC 10649086. PMID 37800975.
Other examples of Mpro inhibitors in late-stage development include STI-1558, currently in the phase 3 clinical trial in adult subjects with mild or moderate COVID-19 (NCT05716425).
- ^ Jump up to:a b c d e f Hackett DW (26 June 2023). “Second Generation Oral Mpro Inhibitor for COVID-19 Treatment Proceeds in Phase 3 Study”. Precision Vaccinations. Retrieved 27 December 2023.
- ^ Jump up to:a b “Coronavirus disease 2019 (COVID-19) emerging treatments”. BMJ Best Practice US. Archived from the original on 27 December 2023. Retrieved 27 December 2023.
- ^ Janin YL (September 2023). “On the origins of SARS-CoV-2 main protease inhibitors”. RSC Medicinal Chemistry. 15 (1): 81–118. doi:10.1039/D3MD00493G. ISSN 2632-8682. PMC 10809347. PMID 38283212. S2CID 264103864.
- ^ Jump up to:a b c d e Mao L, Shaabani N, Zhang X, Jin C, Xu W, Argent C, et al. (January 2024). “Olgotrelvir, a dual inhibitor of SARS-CoV-2 Mpro and cathepsin L, as a standalone antiviral oral intervention candidate for COVID-19”. Med (New York, N.Y.). 5 (1): 42–61.e23. doi:10.1016/j.medj.2023.12.004. PMID 38181791.
- ^ Berdowska I, Matusiewicz M (October 2021). “Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis – with impact on gastrointestinal tract”. World Journal of Gastroenterology. 27 (39): 6590–6600. doi:10.3748/wjg.v27.i39.6590. PMC 8554394. PMID 34754154.
- ^ Jiang R, Han B, Xu W, Zhang X, Peng C, Dang Q, et al. (June 2024). “Olgotrelvir as a Single-Agent Treatment of Nonhospitalized Patients with Covid-19”. NEJM Evidence. 3 (6): EVIDoa2400026. doi:10.1056/EVIDoa2400026. PMID 38804790.
- ^ Sherman AC, Baden LR (June 2024). “How To Measure Benefit in a Changing Pandemic – Olgotrelvir for SARS-CoV-2”. NEJM Evidence. 3 (6): EVIDe2400144. doi:10.1056/EVIDe2400144. PMID 38804789.
- ^ “Sorrento Announces Phase 3 Trial Met Primary Endpoint and Key Secondary Endpoint in Mild or Moderate COVID-19 Adult Patients Treated with Ovydso (Olgotrelvir), an Oral Mpro Inhibitor as a Standalone Treatment for COVID-19” (Press release). BioSpace. 12 September 2023. Retrieved 27 December 2023.
| Clinical data | |
|---|---|
| Trade names | Ovydso |
| Other names | STI-1558, HY-156655, CS-0887294 |
| Routes of administration | By mouth |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 2763596-71-8 |
| PubChem CID | 166157331 |
| UNII | ZP3BDH359D |
| KEGG | D12777 |
| Chemical and physical data | |
| Formula | C22H30N4O7S |
| Molar mass | 494.56 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
//////Olgotrelvir, STI-1558, HY-156655, CS-0887294, STI 1558, HY 156655, CS 0887294, ZP3BDH359D














