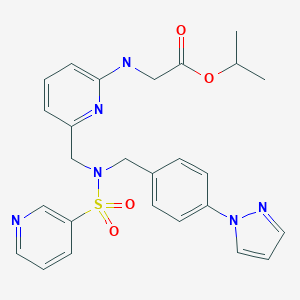

Omidenepag isopropyl
DE-117
Glycine, N-(6-((((4-(1H-pyrazol-1-yl)phenyl)methyl)(3-pyridinylsulfonyl)amino)methyl)-2-pyridinyl)-, 1-methylethyl ester
[[6-[[[4-(Pyrazol-1-yl)benzyl](pyridin-3-ylsulfonyl)amino]methyl]pyridin-2-yl]amino]acetic acid isopropyl ester
C26H28N6O4S, 520.6033, CAS: 1187451-19-9
APPROVED 2018/9/21 PMDA, JAPAN 2018, Eybelis
Antiglaucoma, Prostaglandin E2 receptor agonist, Treatment of Open-Angle Glaucoma and Ocular Hypertension
- Originator Ube Industries
- Developer Santen Pharmaceutical
- Class Eye disorder therapies; Pyrazoles; Pyridines; Small molecules; Sulfonamides
- Mechanism of Action Prostaglandin E EP2 receptor agonists
- Registered Glaucoma; Ocular hypertension
- 27 Sep 2018 Santen initiates enrolment in the phase III Spectrum 5 trial for Glaucoma and Ocular hypertension in USA (Ophthalmic) (NCT03697811)
- 21 Sep 2018 Santen Pharmaceutical and Ube Industries plan phase III trials for omidenepag isopropyl in USA in the second half of 2018
- 21 Sep 2018 Registered for Ocular hypertension and Glaucoma in Japan (Ophthalmic) – First global approval
SYNTHESIS
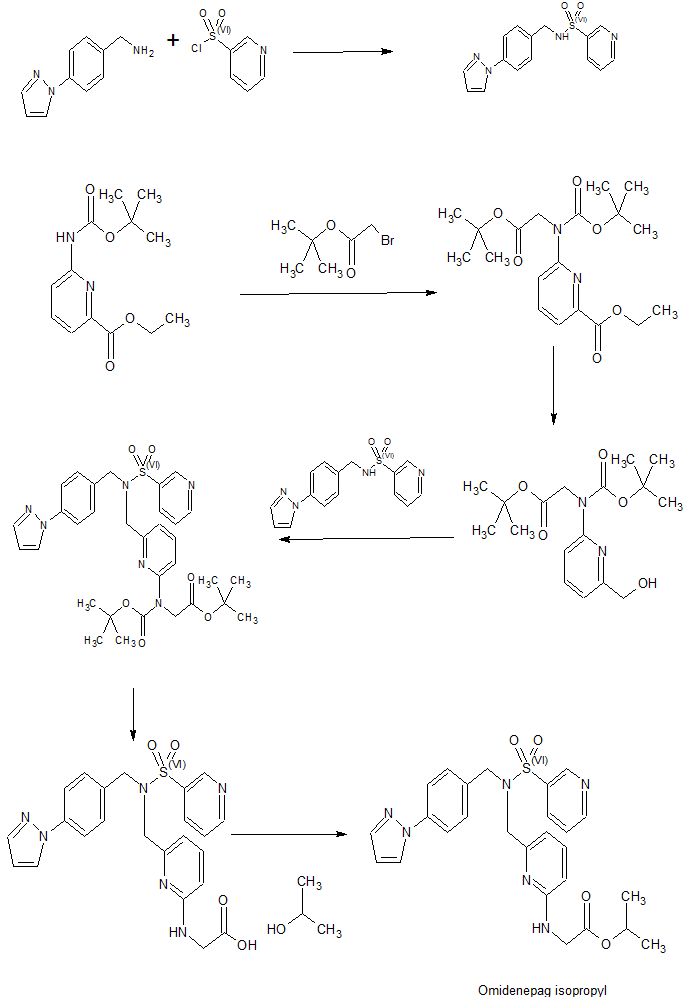
PATENT
WO 2009113600
WO 2010113957
JP 2011057633
PATENT
WO 2015190507
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015190507&tab=FULLTEXT&maxRec=1000


[Example 1]
[Formula

10] 2 – {[6 – ({N-[4-(1H-pyrazol-1-yl) benzyl] pyridin-3-sulfonamido} methyl) pyridin-2-yl] amino} Synthesis of isopropyl acetate
To a glass vessel having an inner volume of about 50 ml equipped with a stirring device, a thermometer and an upper cooling device, 3.21 g (10.00 mmol) of N- [4- (1H-pyrazol-1-yl) benzyl] , 2.43 g (10.0 mmol) of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate obtained in Example 6, 6.65 g (20.4 mmol) of cesium carbonate and 17.6 g of acetonitrile was added, and the mixture was heated and stirred at 80 ° C. In the high performance liquid chromatography analysis, the reaction was carried out for 2 hours until the area percentage of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate became 0.03% or less, I went for hours. The reaction conversion ratios of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate after heating and stirring 1 hour and 2 hours were 99.88% and 99.97% . After completion of the reaction, the reaction solution was cooled to room temperature, filtered using Celite (trade name), and the filtrate was washed with acetonitrile. Quantitative analysis of the obtained filtrate by high performance liquid chromatography revealed that 5.08 g of the objective substance was contained (reaction yield: 97.5%). Next, the reaction solution was concentrated under reduced pressure until the weight of the liquid reached 7.85 g, 42.8 g of toluene was added, and the mixture was washed three times with water. 31.5 ml (31.5 mmol) of 1 mol / L hydrochloric acid was added to the obtained organic layer, and the mixture was stirred at room temperature for 20 minutes and then separated. Note that 0.17 g (corresponding to 3.2% yield) of target product was contained in the organic layer after liquid separation. 42.8 g of toluene and 34.6 ml (34.6 mmol) of 1 mol / L sodium hydroxide aqueous solution were added to the obtained aqueous layer, and the mixture was heated to 40 ° C. and stirred for 20 minutes. After filtration at 40 ° C. in the hot state, liquid separation was carried out. The obtained organic layer was washed twice with water. The organic layer was concentrated under reduced pressure until the weight of the liquid reached 8.97 g, and 7.40 g of 2-propanol was added. After warming to 60 ° C., it was slowly cooled and stirred at 33 ° C. for 30 minutes, then slowly cooled to 5 ° C. or less, and further stirred at the same temperature for 1 hour. Precipitated The solid was filtered, washed with chilled 2-propanol and then vacuum dried at 50 ° C. to give 2 – {[6 – ({N- [4- (1 H-pyrazol- 1 – yl) benzyl] pyridine- 3 – sulfonamido} methyl) pyridin-2-yl] amino} acetic acid was obtained as a slightly brown solid (2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate Isolated yield of standard 75.1%). The quantitative purity by HPLC high performance chromatography method was 99.5%, and 0.04% of N- [4- (1H-pyrazol-1-yl) benzyl] pyridine-3-sulfonamide as a raw material was contained It was. Also, in the measurement (wavelength 260 nm) by the HPLC high performance liquid chromatography method, there was no impurity showing an area% of 0.1% or more.
Physical property values of the obtained 2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine-3-sulfonamido} methyl) pyridin-2- yl] amino} , It was as follows.
EI-MS (m /
z):. 520 [M] CI-MS (m /
[Mz):. + 1] 1 H-NMR (CDCl 3, [delta] (ppm)): 1.24 (6H, d, J = 6.3 Hz), 5.07 (1 H, se, J = 5.5 Hz), 3.82 (2 H, d, J = 5.5 Hz), 4.31 (2 H, s), 4.64 (2 H, s), 4.94 J = 6.3 Hz), 6.26 (1 H, d, J = 8.3 Hz), 6.41 (1 H, dd, J = 7.2, 0.5 Hz), 6.46 (1 H, dd, J = 2.5, 1.8 Hz), 7.25 (2H, m), 7.71 (1H, dd, J = 8.3, 7.2 Hz), 7.32 (1H, ddd, J = 8.0, 4.9, 0.8 Hz), 7.37-7.42 J = 1.8, 0.6 Hz), 7.93 (1 H, dd, J = 2.6, 0.6 Hz), 7.94 (1 H, ddd, J = 8.0, 2.4, 1.7 Hz), 8.69 (1 H, dd, J = 4.8, 1.6 Hz ), 8.98 (IH, dd, J = 2.4, 0.8
Hz). 13 C-NMR (CDCl 3, δ (ppm)): 21.8, 43.7, 51.0, 51.1, 68.9, 107.4, 107.7, 112.6, 119.2, 123.3, 126.7, 129.9, 133.8, 134.6, 137.3, 137.6, 139.8, 141.1, 148.0, 152.6, 153.2, 157.3 , 1737 (C = O), (2981, 2933) (CH), 3437 (NH) , 170.5.
IR (KBr cm -1 ): 764 (CH), 1161 (S = O), 1525 .
elemental analysis; Calcd: C, 59.80%; H, 5.31%; N, 16.07%
Found: C, 59.98%; H, 5.42%; N, 16.14%.
[Example 2]
[Formula

11] 2 – ({6 – [(N-benzyl-3-sulfonamido) methyl] pyridin-2-yl} amino) -acetic acid isopropyl
0.253 g (1.02 mmol) of N-benzylpyridine-3-sulfonamide, 0.253 g (1.02 mmol) of 2- { 0.243 g (1.00 mmol) of isopropyl acetate, 0.665 g (2.04 mmol) of cesium carbonate and 1.76 g of acetonitrile were added, and the mixture was heated and stirred at 80 ° C. did. In the high performance liquid chromatography analysis, the reaction was carried out for 2 hours until the area percentage of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate became 0.03% or less, I went for hours. The reaction conversion rates of 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetic acid isopropyl acetate after 1 hour and 2 hours from the start of heating and stirring were 99.81% and 99.99% It was. After completion of the reaction, the reaction solution was cooled to room temperature, filtered using Celite (trade name), and the filtrate was washed with acetonitrile. Quantitative analysis of the obtained filtrate by high-performance liquid chromatography revealed that 0.430 g of the target product was contained (reaction yield: 94.5%). Next, the reaction solution was concentrated under reduced pressure until the weight of the liquid reached 0.785 g, 4.3 g of toluene was added, and the mixture was washed three times with water. At this time, an emulsion containing the desired product was produced, but it was discarded together with the aqueous layer. 3.15 ml (3.15 mmol) of 1 mol / L hydrochloric acid was added to the obtained organic layer, and the mixture was stirred at room temperature for 20 minutes and then separated. To the obtained aqueous layer, 4.27 g of toluene and 3.46 ml (3.46 mmol) of 1 mol / L sodium hydroxide aqueous solution were added, the mixture was heated to 40 ° C. and stirred for 20 minutes. After separation, the obtained organic layer was washed twice with water. The organic layer was concentrated under reduced pressure to a liquid weight of 0.239 g to obtain isopropyl 2 – ({6 – [(N-benzylpyridine-3-sulfonamido) methyl] pyridin-2-yl} amino) acetate as a light brown solid (Obtained as a raw material based on isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate Rate 53.8%). The quantitative purity by HPLC high performance liquid chromatography method was 98.0%. Also, in the measurement (wavelength 260 nm) by the HPLC high performance liquid chromatography method, there was no impurity showing an area% of 0.1% or more.
Physical property values of the obtained 2 – ({6 – [(N-benzylpyridine-3-sulfonamido) methyl] pyridin-2-yl} amino) acetate isopropylate were as follows.
EI-MS (m /
z):. 454 [M] CI-MS (m /
[Mz):. + 1] 1 H-NMR (CDCl 3 , [delta] (ppm)): 1.27 (6H, d, J = 5.3 Hz), 5.09 (1 H, sep, J = 6.3 Hz), 3.82 (2H, d, J = 5.4 Hz), 4.31 (2H, s), 4.62 7.26 – 7.33 (7 H, m), 7.90 – 7.93 (1 H, m), 8.69 (1 H, m), 6.26 (1 H, d, J = 8.3 Hz)
13 C-NMR (CDCl 3 , δ (ppm)): 21.8, 43.8, 51.1, 51.6, 69.0, 1 H, dd, J = 4.8, 1.6 Hz), 8.95 (1 H, dd, J = 107.2, 112.6, 123.2, 127.9, 128.6, 128.8, 134.7, 135.6, 137.6, 137.7, 148.2, 152.5, 153.6, 157.3, 170.5
IR (KBr cm -1
Calcd: C, 60.77%; H, 5.77%; N, 12.33%
Found (C = : C, 61.03%; H, 5.85%; N, 12.15%.
Example 3 Synthesis
of

2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine-3-sulfonamido} methyl) pyridin- Synthesis of isopropyl acetate
641 mg (2.04 mmol) of N- [4- (1H-pyrazol-1-yl) benzyl] pyridine-3-sulfonamide was added to a glass container having an inner volume of about 30 ml equipped with a stirrer, a thermometer and an upper cooling device, , 485 mg (2.00 mmol) of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate obtained in Example 6, 1.33 g (4.08 mmol) of cesium carbonate and 3.53 g And the mixture was stirred at 30 ° C. The reaction was carried out for 26 hours until the area percentage of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate was 0.3% or less in the high performance liquid chromatography analysis, I went for hours. After completion of the reaction, the reaction solution was filtered, and the filtrate was washed with acetonitrile. Quantitative analysis of the obtained filtrate by high performance liquid chromatography showed that 991 mg of the desired product was contained (reaction yield 95.2%).
Example 4
Synthesis of Isopropyl Acetate of 2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine-3-sulfonamido) methyl)
To a glass container having an inner volume of about 50 ml equipped with a stirring device, a thermometer and an upper cooling device, 3.21 g (10.00 mmol) of N- [4- (1H-pyrazol-1-yl) benzyl] 2.43 g (10.0 mmol) of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate obtained in Example 6, 2.82 g (20.4 mmol) potassium carbonate obtained in Example 6 and 17.6 g of acetonitrile was added, and the mixture was heated and stirred at 80 ° C. The reaction was carried out for 10 hours in the high performance liquid chromatography analysis until the area percentage of isopropyl 2 – {[6- (chloromethyl) pyridin-2-ylamino] acetate as raw material was 0.03% or less. The reaction conversion rate of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate was 43.9% after 1 hour from the start of heating and stirring. After completion of the reaction, the reaction solution was cooled to room temperature, filtered using Celite (trade name), and the filtrate was washed with acetonitrile. Quantitative analysis of the obtained filtrate by high performance liquid chromatography revealed that 5.00 g of the target product was contained (reaction yield 96.0%). Next, the reaction solution was concentrated under reduced pressure until the weight of the liquid reached 7.85 g, 42.77 g of toluene was added, and then washed three times with water. 31.5 ml (31.5 mmol) of 1 mol / L hydrochloric acid was added to the obtained organic layer, and the mixture was stirred at room temperature for 20 minutes and then separated. Incidentally, 0.62 g (corresponding to a yield of 11.8%) of the target product was contained in the organic layer after liquid separation. 42.77 g of toluene and 34.6 ml (34.6 mmol) of a 1 mol / L sodium hydroxide aqueous solution were added to the obtained aqueous layer, and the mixture was heated to 40 ° C. and stirred for 20 minutes. After filtration at 40 ° C. in the hot state, liquid separation was carried out. The obtained organic layer was washed twice with water. The organic layer was concentrated under reduced pressure until the weight of the liquid reached 8.97 g, and 7.40 g of 2-propanol was added. After heating to 60 ° C., it was slowly cooled and stirred at a temperature at which crystal began to precipitate for 30 minutes, then slowly cooled to 5 ° C. or less, and stirred at the same temperature for 1 hour. The obtained slurry was filtered, and the obtained filtrate was washed with water After washing with cooled 2-propanol and vacuum drying at 50 ° C., 2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine- Methyl) pyridin-2-yl] amino} acetic acid 3.90 g as a slightly brown solid (isolation based on isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate Rate 74.9%). The quantitative purity by HPLC high performance chromatography method was 99.0%, and 0.11% of N- [4- (1H-pyrazol-1-yl) benzyl] pyridine-3-sulfonamide as a raw material was contained It was.
Physical property values of the obtained 2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine-3-sulfonamido} methyl) pyridin-2- yl] amino} , It was as follows.
EI-MS (m /
z):. 520 [M] CI-MS (m /
[Mz):. + 1] 1 H-NMR (CDCl 3, [delta] (ppm)): 1.24 (6H, d, J = 6.3 Hz), 5.07 (1 H, se, J = 5.5 Hz), 3.82 (2 H, d, J = 5.5 Hz), 4.31 (2 H, s), 4.64 (2 H, s), 4.94 J = 6.3 Hz), 6.26 (1 H, d, J = 8.3 Hz), 6.41 (1 H, dd, J = 7.2, 0.5 Hz), 6.46 (1 H, dd, J = 2.5, 1.8 Hz), 7.25 (2H, m), 7.71 (1H, dd, J = 8.3, 7.2 Hz), 7.32 (1H, ddd, J = 8.0, 4.9, 0.8 Hz), 7.37-7.42 J = 1.8, 0.6 Hz), 7.93 (1 H, dd, J = 2.6, 0.6 Hz), 7.94 (1 H, ddd, J = 8.0, 2.4, 1.7 Hz), 8.69 (1 H, dd, J = 4.8, 1.6 Hz ), 8.98 (IH, dd, J = 2.4, 0.8
Hz). 13 C-NMR (CDCl 3, δ (ppm)): 21.8, 43.7, 51.0, 51.1, 68.9, 107.4, 107.7, 112.6, 119.2, 123.3, 126.7, 129.9, 133.8, 134.6, 137.3, 137.6, 139.8, 141.1, 148.0, 152.6, 153.2, 157.3 , 1737 (C = O), (2981, 2933) (CH), 3437 (NH) , 170.5.
IR (KBr cm -1 ): 764 (CH), 1161 (S = O), 1525 .
elemental analysis; Calcd: C, 59.80%; H, 5.31%; N, 16.07%
Found: C, 59.98%; H, 5.42%; N, 16.14%.
Comparative Example 1
Synthesis of Isopropyl Acetate of 2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine-3-sulfonamido} methyl)
To a glass vessel having an inner volume of about 50 ml equipped with a stirring device, a thermometer and an upper cooling device, 3.21 g (10.00 mmol) of N- [4- (1H-pyrazol-1-yl) benzyl] , 2.43 g (10.0 mmol) of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate obtained in Example 6, 2.16 g (20.4 mmol) of sodium carbonate and 17.6 g of acetonitrile was added, and the mixture was heated and stirred at 80 ° C. In the high performance liquid chromatography analysis, the reaction was carried out for 110 hours until the area percentage of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate became 0.05% or less. The reaction conversion rate of isopropyl 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate 1 hour after the start of heating and stirring was 0.92%. After completion of the reaction, the reaction solution was cooled to room temperature, filtered using Celite (trade name), and the filtrate was washed with acetonitrile. Quantitative analysis of the obtained filtrate by high performance liquid chromatography revealed that 0.72 g of the target product was contained (reaction yield: 13.8%). Next, the solution was concentrated under reduced pressure until the weight of the solution reached 7.85 g, 42.6 g of toluene was added, and the mixture was washed three times with water. Since the tar component was separated at the time of washing with water, it was discarded together with the aqueous layer. 31.5 ml (31.5 mmol) of 1 mol / L hydrochloric acid was added to the obtained organic layer, and the mixture was stirred at room temperature for 20 minutes and then separated. 42.6 g of toluene and 34.6 ml (34.6 mmol) of 1 mol / L sodium hydroxide aqueous solution were added to the obtained aqueous layer, and the mixture was heated to 40 ° C. and stirred for 20 minutes. After filtration at 40 ° C. in the hot state, liquid separation was carried out, and the obtained organic layer was washed twice with water. The organic layer was concentrated under reduced pressure to give isopropyl acetate (2 – {[6 – ({N- [4- (1 H-pyrazol- 1 – yl) benzyl] pyridine- To obtain a dark brown viscous liquid containing 0.764. The quantitative purity by HPLC high performance chromatography method was 60.2%, the pure content was 0.
Physical property values of the obtained 2 – {[6 – ({N- [4- (1 H-pyrazol-1 -yl) benzyl] pyridine-3-sulfonamido} methyl) pyridin-2- yl] amino} , It was as follows.
EI-MS (m /
z):. 520 [M] CI-MS (m /
[Mz):. + 1] 1 H-NMR (CDCl 3, [delta] (ppm)): 1.24 (6H, d, J = 6.3 Hz), 5.07 (1 H, se, J = 5.5 Hz), 3.82 (2 H, d, J = 5.5 Hz), 4.31 (2 H, s), 4.64 (2 H, s), 4.94 J = 6.3 Hz), 6.26 (1 H, d, J = 8.3 Hz), 6.41 (1 H, dd, J = 7.2, 0.5 Hz), 6.46 (1 H, dd, J = 2.5, 1.8 Hz), 7.25 (2H, m), 7.71 (1H, dd, J = 8.3, 7.2 Hz), 7.32 (1H, ddd, J = 8.0, 4.9, 0.8 Hz), 7.37-7.42 J = 1.8, 0.6 Hz), 7.93 (1 H, dd, J = 2.6, 0.6 Hz), 7.94 (1 H, ddd, J = 8.0, 2.4, 1.7 Hz), 8.69 (1 H, dd, J = 4.8, 1.6 Hz ), 8.98 (IH, dd, J = 2.4, 0.8
Hz). 13 C-NMR (CDCl 3, δ (ppm)): 21.8, 43.7, 51.0, 51.1, 68.9, 107.4, 107.7, 112.6, 119.2, 123.3, 126.7, 129.9, 133.8, 134.6, 137.3, 137.6, 139.8, 141.1, 148.0, 152.6, 153.2, 157.3 , 1737 (C = O), (2981, 2933) (CH), 3437 (NH) , 170.5.
IR (KBr cm -1 ): 764 (CH), 1161 (S = O), 1525 .
Example 5
Synthesis of 2 – {[6- (hydroxymethyl) pyridin-2-yl] amino} acetate isopropylate
948 g of 2-propanol and 76.7 g of concentrated sulfuric acid were added to a glass container having an inner volume of about 2 L and equipped with a stirring device, a thermometer and an upper cooling device, and the mixture was heated to 75 ° C. To this was added 2 – {[(t-butoxycarbonyl) (6-hydroxymethylpyridin-2-yl)] amino} acetic acid tert- butyl ester synthesized by the method described in Reference Example 3- (b) A mixed solution of 135 g of butyl, 45 g of toluene and 311 g of 2-propanol was added dropwise over 40 minutes, followed by heating and stirring at 78 ° C. for 6 hours. After cooling, 677 g of toluene and 406 g of water were added under an internal pressure of 20 hPa and an external temperature of 40 ° C. until the amount of liquid reached 309 g, and the mixture was stirred at room temperature and then separated. The obtained aqueous layer was added dropwise to a mixed solution of 129 g of separately prepared sodium hydrogencarbonate, 812 g of water, and 677 g of toluene over 20 minutes, stirred at room temperature for 1 hour, separated, and the aqueous layer was washed with 338 g . The obtained organic layer was mixed and washed with 426 g of a 5 wt% sodium chloride aqueous solution to obtain 1370 g of an organic layer. Approximately 1356 g of this was taken out, concentrated to a liquid volume of 113 g, and then toluene was added until the liquid amount reached 300 g. 190 g of n-heptane was added to the solution, and the solution was warmed to 45 ° C. to dissolve the crystals, followed by cooling to 35 ° C. A small amount of separately synthesized seed crystals was added in the same way and stirred at 35 ° C. for 1 hour, the crystals gradually increased. 365 g of n-heptane was added dropwise over 30 minutes, cooled for 40 minutes until the internal temperature reached 5 ° C., and stirred at the same temperature for 30 minutes. The precipitated crystals were separated by filtration, washed with n-heptane and then dried under reduced pressure at 50 ° C. to obtain 70.4 g of isopropyl 2 – {[6- (hydroxymethyl) pyridin-2-yl] amino} . The quantitative purity by HPLC high performance chromatography was 94.3%, and the pure content was 66.4 g (raw material 2 – {[(t-butoxycarbonyl) (6-hydroxymethylpyridin-2-yl )] Amino} acetate as t-butyl acetate in an isolated yield of 74.7%).
Physical properties of the obtained 2 – {[6- (hydroxymethyl) pyridin-2-yl] amino} acetate isopropyl were as follows.
EI-MS (m /
z):. 224 [M] CI-MS (m /
[Mz):. + 1] 1 H-NMR (CDCl 3, [delta] (ppm)): 1.27 (6H, d, J = 6.3 Hz), 3.76 (IH, s), 4.10 (2H, d, J = 5.5 Hz), 4.59 (2H, s), 5.00 (IH, s), 5.10 (IH, m), 6.36
13 C-NMR (CDCl 3, δ (ppm) ), 6.51 (1 H, dd, J = 7.3, 0.7 Hz), 7.41 (1 H, ddd, J = 5.74, 3.88 Hz ) ): 21.8, 44.1, 63.5, 69.0, 106.6, 109.5, 138.0, 156.8, 156.9, 170.7
IR (KBr cm -1): 416, 469, 531, 559, 731, 785, 826, 862, 903, 916, 941, 980, 1014, 1052, 1082, 1106, 1131, 1147, 1182, 1217, 1256, 1276, 1347, 1378,
Calcd: C, 58.91% Calcd: C, 58.91% (C = O) ; H, 7.19%; N, 12.49%
Found: C, 58.99%; H, 7.17%; N, 12.48%.
Example 6
Synthesis of 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate isopropylate
To a solution of 35.7 g of isopropyl 2 – {[6- (hydroxymethyl) pyridin-2-yl] amino} acetate obtained in Example 5 in 396 g of methylene chloride was added 19.6 g of thionyl chloride at room temperature Was added dropwise over 20 minutes, and the mixture was stirred at room temperature for 1 hour. The obtained reaction solution was added dropwise to a mixed liquid slurry of 37.8 g of sodium hydrogencarbonate and 149 g of water, and the mixture was stirred at room temperature for 20 minutes. After liquid separation, 6.73 g of magnesium sulfate was added to the organic layer, dehydrated and the filtrate was concentrated to dryness at 50 ° C. to obtain 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate 37 .8 g as a light brown solid.
Physical properties of the obtained 2 – {[6- (chloromethyl) pyridin-2-yl] amino} acetate isopropyl were as follows.
EI-MS (m /
z):. 242 [M] CI-MS (m /
[Mz):. + 1] 1 H-NMR (CDCl 3, [delta] (ppm)): 1.24 (6H, m) J = 8.3 Hz), 4.7 (2H, d, J = 5.4 Hz), 4.48 (2H, s), 5.03 (IH, s), 5.10 (IH, m), 6.39
13 C-NMR (CDCl 3, δ (ppm)): 21.8, 44.0, 44.7, 68.9, 107.7, 112.2, 138.1, 1 H NMR (CDCl 3, δ (ppm)): 7.43 (1H, dd, J = 7.8, 7.8 Hz)154.6, 157.3, 170.7
IR (KBr cm -1): 415, 446, 530, 560, 627, 735, 804, 827, 874, 903, 939, 952, 982, 1042, 1088, 1108, 1128, 1144, 1167, 1180, 1219, 1269, 1281, 1350,
Elemental analysis: 1378, 1400, 1420, 1434, 1470, 1525 (C = N), 1580, 1613, 1690, 1728 (C = O), 2878, 2934 (CH), 2981 (CH), 3379Calcd: C, 54.44%; H, 6.23%; N, 11.54%
Found: C, 54.46%; H, 6.23%; N, 11.56%.
PAPER
Journal of Medicinal Chemistry (2018), 61(15), 6869-6891.
Identification of a Selective, Non-Prostanoid EP2 Receptor Agonist for the Treatment of Glaucoma: Omidenepag and its Prodrug Omidenepag Isopropyl
† Pharmaceuticals Research Laboratory, UBE Industries, Ltd., 1978-5 Kogushi, Ube, Yamaguchi 755-8633, Japan
‡ R&D Division, Santen Pharmaceutical Co., Ltd., Grand Front Osaka Tower A 4-20, Ofukacho, Kita-ku, Osaka 530-8552, Japan
§ R&D Division, Santen Inc., 6401 Hollis Street, Suite 125, Emeryville, California 94608, United States
J. Med. Chem., 2018, 61 (15), pp 6869–6891
DOI: 10.1021/acs.jmedchem.8b00808

EP2 receptor agonists are expected to be effective ocular hypotensive agents; however, it has been suggested that agonism to other EP receptor subtypes may lead to undesirable effects. Through medicinal chemistry efforts, we identified a scaffold bearing a (pyridin-2-ylamino)acetic acid moiety as a promising EP2-selective receptor agonist. (6-((4-(Pyrazol-1-yl)benzyl)(pyridin-3-ylsulfonyl)aminomethyl)pyridin-2-ylamino)acetic acid 13ax (omidenepag, OMD) exerted potent and selective activity toward the human EP2 receptor (h-EP2). Low doses of omidenepag isopropyl (OMDI), a prodrug of 13ax, lowered intraocular pressure (IOP) in ocular normotensive monkeys. OMDI was selected as a clinical candidate for the treatment of glaucoma.
Isopropyl (6-((4-(Pyrazol-1-yl)benzyl)(pyridin-3-ylsulfonyl)aminomethyl)pyridin-2- ylamino)acetate (OMDI)
white solid. 1H NMR (500 MHz, DMSO-d6) δ 8.87 (dd, J = 2.4, 0.7 Hz, 1H), 8.75 (dd, J = 4.8, 1.6 Hz, 1H), 8.48 (dd, J = 2.4, 0.5 Hz, 1H), 8.08 (ddd, J = 8.1, 2.4, 1.6 Hz, 1H), 7.80–7.77 (m, 2H), 7.74 (dd, J = 1.8, 0.5 Hz, 1H), 7.51 (ddd, J = 8.1, 4.8, 0.7 Hz, 1H), 7.36–7.33 (m, 2H), 7.26 (dd, J = 8.3, 7.1 Hz, 1H), 6.89 (t, J = 6.1, 1H), 6.54 (dd, J = 2.4, 1.8 Hz, 1H), 6.38 (d, J = 8.3 Hz, 1H), 6.34 (d, J = 7.1 Hz, 1H), 4.87 (sept, J = 6.3 Hz, 1H), 4.62 (s, 2H), 4.21(s, 2H), 3.76 (d, J = 6.1 Hz, 2H), 1.10 (d, J = 6.3 Hz, 6H). 13C NMR (proton-decoupled spectrum, 500 MHz, DMSO-d6) δ 171.2 (s), 158.1 (s), 153.4 (s), 153.2 (s), 147.6 (s), 141.4 (s), 139.6 (s), 137.5 (s), 137.0 (s), 135.1 (s), 134.4 (s), 129.9 (s), 128.2 (s), 124.4 (s), 118.8 (s), 111.4 (s), 108.3 (assigned for two nonequivalent carbons with identical chemical shift), 68.0 (s), 51.9 (s), 51.2 (s), 43.1 (s), 22.0 (s). MS (CI+) m/z521 (M + H)+. IR wavelength [cm–1] 3437 (N–H), 1736 (C═O), 1608, 1525, and 1511 (C═C and C═N), 1321 (SO2), 1161 (SO2). Elemental analysis [%] (average of three experiments) calculated for C26H28N6O4S: C 59.98, H 5.42, N 16.14. Found: C 59.76, H 5.28, N 16.01. TLC Rf value 0.39 (ethyl acetate).
//////////////Omidenepag isopropyl, JAPAN 2018, オミデネパグイソプロピル , DE-117, UBE, SANTEN
CC(C)OC(=O)CNc1cccc(CN(Cc2ccc(cc2)n3cccn3)S(=O)(=O)c4cccnc4)n1


















