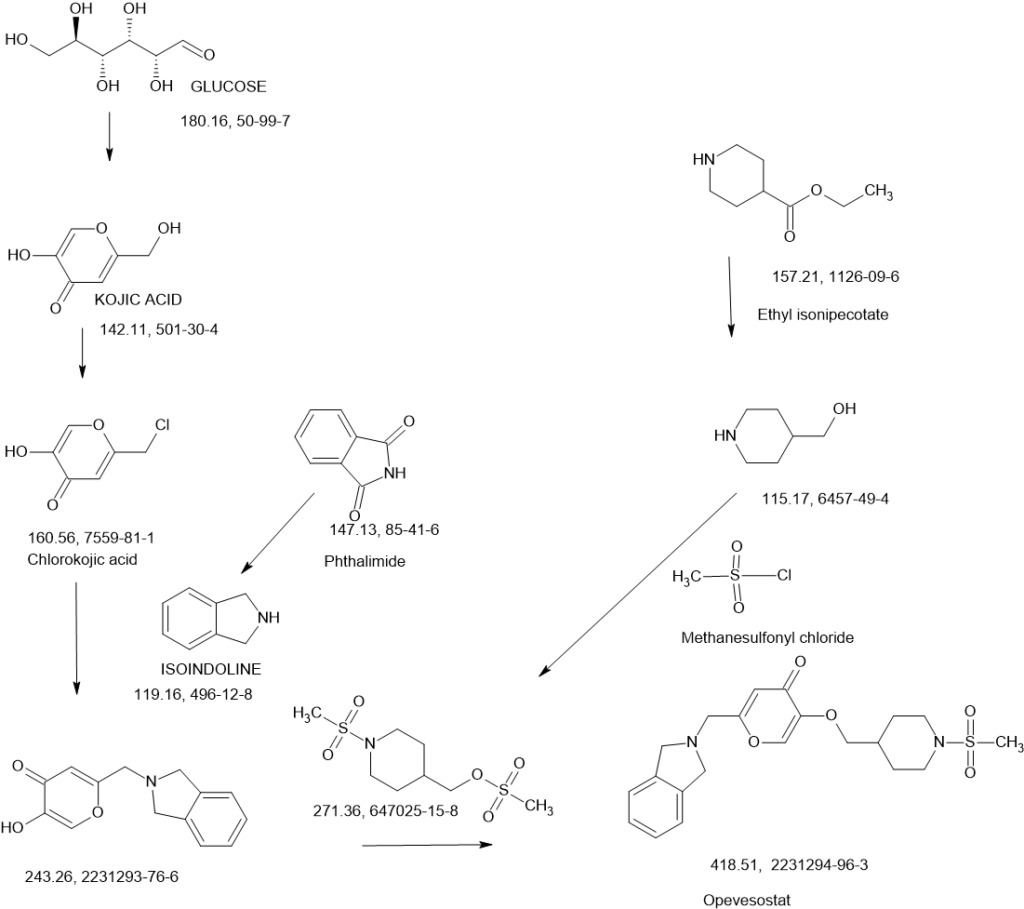
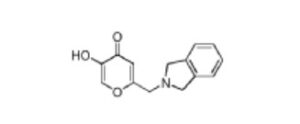
Opevesostat
- ODM208
2231294-96-3
Chemical Formula: C21H26N2O5S
Molecular Weight: 418.508
2-[(1,3-dihydro-2H-isoindol-2-yl)methyl]-5-{[1-(methanesulfonyl)piperidin-4-yl]methoxy}-4H-pyran-4-one
| Opevesostat tosylate |
4H-Pyran-4-one, 2-[(1,3-dihydro-2H-isoindol-2-yl)methyl]-5-[[1-(methylsulfonyl)-4-piperidinyl]methoxy]-, 4-methylbenzenesulfonate (1:1)
2-((1,3-DIHYDRO-2H-ISOINDOL-2-YL)METHYL)-5-((1-(METHYLSULFONYL)-4-PIPERIDINYL)METHOXY)-4H-PYRAN-4-ONE, TOSYLATE
SCHEME
SEE AT THE END OF PAGE
Opevesostat is an investigational new drug being developed for the treatment of metastatic castration-resistant prostate cancer (mCRPC).[1] It is a non-steroidal, selective inhibitor of CYP11A1 (cholesterol side-chain cleavage enzyme)[2] discovered by Orion Corporation and currently undergoing clinical development by Merck & Co., Inc. Opevesostat’s mechanism of action involves suppressing the production of steroid hormones and their precursors that may activate the androgen receptor signaling pathway, which is crucial in prostate cancer progression. As of 2024, opevesostat is being evaluated in two Phase 3 clinical trials, OMAHA1 and OMAHA2a, which are assessing its efficacy and safety in combination with hormone replacement therapy for patients with mCRPC who have failed prior treatments.[3][4][5]
useful in the treatment of a steroid receptor, in particular androgen receptor (AR), dependent conditions and diseases, and to pharmaceutical compositions containing such compounds.
Prostate cancer is worldwide the most common cancer in men. Even though the 5-year survival rate of patients with localized prostate cancer is high, the prognosis for those patients, who develop castration-resistant prostate cancer (CRPC) within that 5-year follow-up period, is poor.
The androgen receptor (AR) signalling axis is critical in all stages of prostate cancer. In the CPRC stage, disease is characterized by high AR expression, AR amplification and persistent activation of the AR signalling axis by residual tissue/tumor androgens and by other steroid hormones and intermediates of steroid biosynthesis. Thus, treatment of advanced prostate cancer involves androgen deprivation therapy (ADT) such as hormonal manipulation using gonadotropin-releasing hormone (GnRH) agonists/antagonists or surgical castration, AR antagonists or CYP17A1 inhibitors (such as abiraterone acetate in combination with prednisone).
Although therapies can initially lead to disease regression, eventually majority of the patients develop a disease that is refractory to currently available therapies. Increased progesterone levels in patients treated with abiraterone acetate has been hypothesized to be one of the resistance mechanisms. Several nonclinical and clinical studies have indicated upregulation of enzymes that catalyse steroid biosynthesis at the late stage of CRPC. Very recently it has been published that 11β-OH androstenedione can be
metabolized into 11-ketotestosterone (11-K-T) and 11-ketodehydrotestosterone (11-K-DHT) which can bind and activate AR as efficiently as testosterone and dihydrotestosterone. It has been shown that these steroids are found in high levels in plasma and tissue in prostate cancer patients, suggesting their role as AR agonists in CRPC. Furthermore, it has been addressed that prostate cancer resistance to CYP17A1 inhibition may still remain steroid dependent and responsive to therapies that can further suppress de novo intratumoral steroid synthesis upstream of CYP17A1, such as by CYP11A1 inhibition therapy (Cai, C. et al, Cancer Res., 71(20), 6503-6513, 2011).
Cytochrome P450 monooxygenase 11A1 (CYP11A1), also called cholesterol side chain cleavage enzyme, is a mitochondrial monooxygenase which catalyses the conversion of cholesterol to pregnenolone, the precursor of all steroid hormones. By inhibiting CYP11A1, the key enzyme of steroid biosynthesis upstream of CYP17A1, the total block of the whole steroid biosynthesis can be achieved. CYP11A1 inhibitors may therefore have a great potential for treating steroid hormone dependent cancers, such as prostate cancer, even in advanced stages of the disease, and especially in those patients who appear to be hormone refractory. It has been recently shown that a compound having CYP11A1 inhibitory effect significantly inhibited tumor growth in vivo in a murine CRPC xenograft model (Oksala, R. et al, Annals of Oncology, (2017) 28 (suppl.
PATENT
WO2018115591
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018115591&_cid=P20-LQXJT7-60871-1
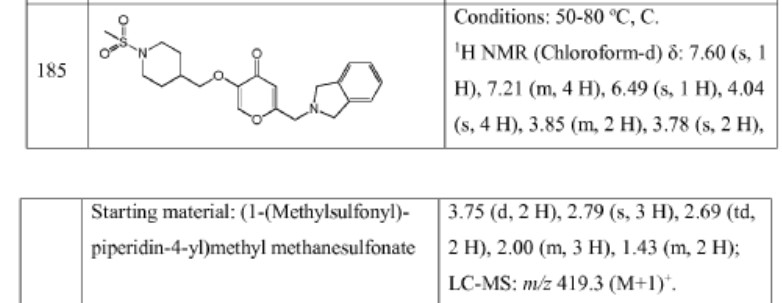
Example 4. SIMILAR
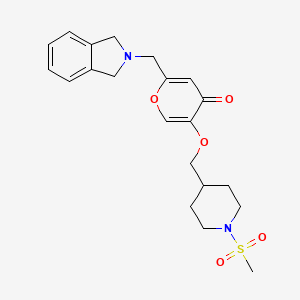
N-((4-(((6-(Isoindolin-2-ylmethyl)-4-oxo-4H-pyran-3-yl)oxy)methyl)cyclohexyl)- methyl)methanesulfonamide (Compound 173)
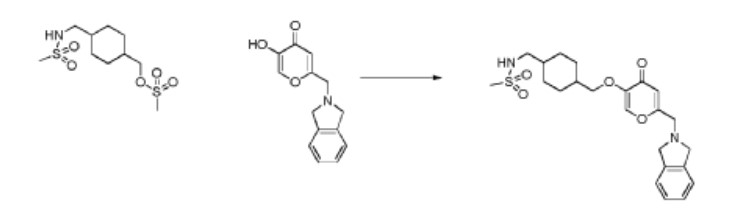
To a solution of 5-hydroxy-2-(isoindolin-2-ylmethyl)-4H-pyran-4-one (0.10 g, 0.41 mmol) in DMF (2 ml) were added (4-(methylsulfonamidomethyl)cyclohexyl)methyl methanesulfonate (0.14 g, 0.45 mmol) and K2CO3 (0.12 g, 0.8 mmol). The reaction mixture was heated at 80 °C for 2 h. The mixture was cooled to RT, water (10 ml) was added and the product was extracted with EtOAc. The combined extracts were washed with water, dried with Na2SO4, filtered and evaporated. The crude product was purified by column chromatography to afford the title compound (0.06 g). 1H NMR (400 MHz, Chloroform-d) δ ppm 0.92 – 1.11 (m, 4 H) 1.40 – 1.63 (m, 2 H) 1.78 – 2.00 (m, 4 H) 2.91 – 2.99 (m, 5 H) 3.65 (d, J=6.46 Hz, 2 H) 3.77 (s, 2 H) 4.03 (s, 4 H) 5.04 (br t, J=6.31 Hz, 1 H) 6.49 (s, 1 H) 7.20 (s, 4 H) 7.59 (s, 1 H).
ntermediate 58: 5-Hydroxy-2-(isoindolin-2-ylmethyl)-4H-pyran-4-one
To a stirred solution of 2-(chloromethyl)-5-hydroxy-4H-pyran-4-one (2.0 g, 12.5 mmol) in acetonitrile (50 mL) were added DIPEA (3.22 mL, 25.0 mmol) and isoindoline (1.78 g, 25.0 mmol) at RT. When the reaction was complete, the precipitated solid was filtered and washed with EtOAc. The title compound was collected as pale brown solid (1.1 g). LC-MS: m/z 244.1 (M+H)+.
///////////////////


AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
References
[edit]- ^ “Opevesostat – Orion”. AdisInsight.
- ^ Kim C, Jeong E, Lee YB, Kim D (July 2024). “Steroidogenic cytochrome P450 enzymes as drug target”. Toxicological Research. 40 (3): 325–333. Bibcode:2024ToxRe..40..325K. doi:10.1007/s43188-024-00237-0. PMC 11187042. PMID 38911541.
- ^ “Merck and Orion Announce Mutual Exercise of Option Providing Merck Global Exclusive Rights to Opevesostat”. StockTitan. July 2024. Retrieved 2024-11-23.
- ^ “Inside Information: Orion and MSD Announce Mutual Exercise of Option Providing MSD Global Exclusive Rights to Opevesostat”. Orion. Retrieved 2024-11-23.
- ^ “Merck and Orion Announce Mutual Exercise of Option Providing Merck Global Exclusive Rights to Opevesostat”. Merck. Retrieved 2024-11-23.
 |
|
| Clinical data | |
|---|---|
| Other names | MK-5684, ODM-208 |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H26N2O5S |
| Molar mass | 418.51 g·mol−1 |
| 3D model (JSmol) | |
//////////Opevesostat, ODM 208, MK-5684, ODM-208, MK 5684
/////////Opevesostat, ODM 208
O=C1C=C(CN2CC3=C(C=CC=C3)C2)OC=C1OCC4CCN(S(=O)(C)=O)CC4