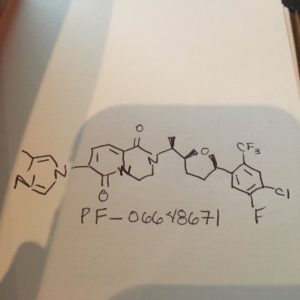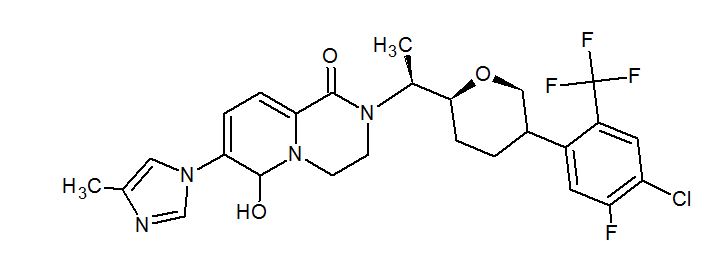
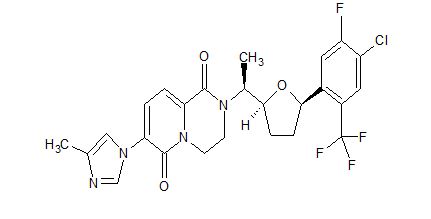
PF-06648671, PF 06648671, PF-6648671
Phase I Alzheimer’s disease
Originator Pfizer
- 01 Nov 2016 Pfizer completes a phase I pharmacokinetics trial in Healthy volunteers in USA (PO) (NCT02883114)
- 01 Oct 2016 Pfizer completes a phase I trial in Healthy volunteers in Belgium (NCT02440100)
- 01 Sep 2016 Pfizer initiates a phase I pharmacokinetics trial in Healthy volunteers in USA (PO) (NCT02883114)

SYNTHESIS
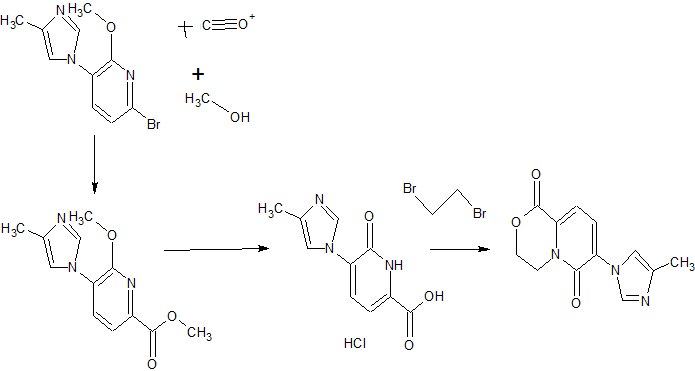
FIRST KEY INTERMEDIATE
CONTD………….
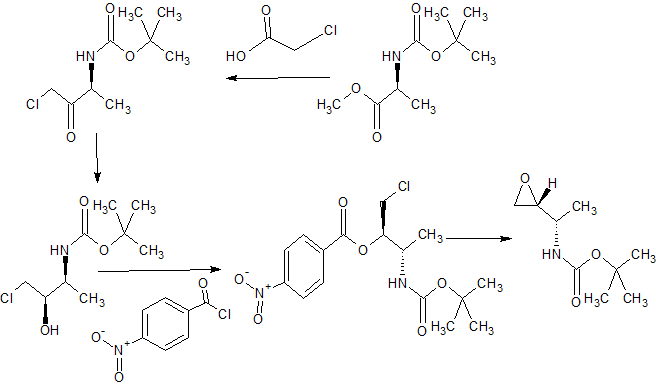
SECOND KEY INTERMEDIATE
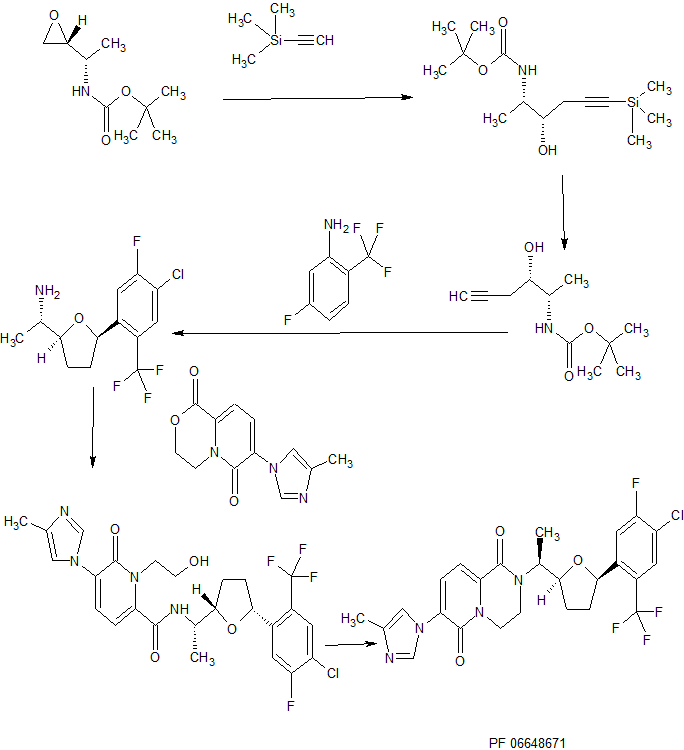
contd……………..
Dementia results from a wide variety of distinctive pathological processes. The most common pathological processes causing dementia are Alzheimer’s disease (AD), cerebral amyloid angiopathy (CM) and prion-mediated diseases (see, e.g., Haan et al., Clin. Neurol. Neurosurg. 1990, 92(4):305-310; Glenner et al., J. Neurol. Sci. 1989, 94:1 -28). AD affects nearly half of all people past the age of 85, the most rapidly growing portion of the United States population. As such, the number of AD patients in the United States is expected to increase from about 4 million to about 14 million by 2050.
The present invention relates to a group of γ-secretase modulators, useful for the treatment of neurodegenerative and/or neurological disorders such as Alzheimer’s disease and Down’s Syndrome, (see Ann. Rep. Med. Chem. 2007, Olsen et al., 42: 27-47).
PATENT
Preparations
Preparation P1 : 5-(4-Methyl-1 H-imidazol-1 -yl)-6-oxo-1 ,6-dihvdropyridine-2-carboxylic
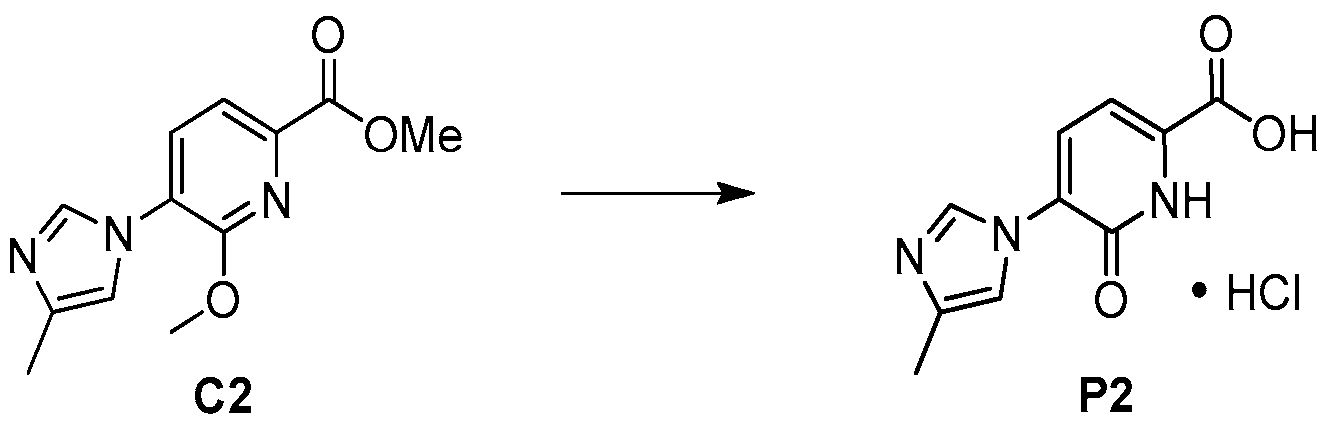
Step 1 . Synthesis of methyl 6-methoxy-5-(4-methyl-1 /-/-imidazol-1 -yl)pyridine-2- carboxylate (C2).
To a solution of the known 6-bromo-2-methoxy-3-(4-methyl-1 /-/-imidazol-1 – yl)pyridine (C1 , T. Kimura et al., U.S. Pat. Appl. Publ. 2009, US 20090062529 A1 ) (44.2 g, 165 mmol) in methanol (165 ml_) was added triethylamine (46 ml_, 330 mmol) and [1 ,1 ‘-bis(diphenylphosphino)ferrocene]dichloropalladium(ll), dichloromethane complex (6.7 g, 8.2 mmol). The mixture was degassed several times with nitrogen. The reaction was heated to 70 °C under CO atmosphere (3 bar) in a Parr apparatus. After 30 minutes, the pressure dropped to 0.5 bar; additional CO was added until the pressure stayed constant for a period of 30 minutes. The mixture was allowed to cool to room temperature and filtered through a pad of Celite. The Celite pad was washed twice with methanol and the combined filtrates were concentrated under reduced pressure. The residue (88 g) was dissolved in ethyl acetate (1 L) and water (700 mL); the organic layer was washed with water (200 mL), and the aqueous layer was extracted with ethyl acetate (500 mL). The combined organic layers were dried over magnesium sulfate, filtered and concentrated to provide the title compound. Yield: 42.6 g, quantitative.
Step 2. Synthesis of 5-(4-methyl-1 H-imidazol-1 -yl)-6-oxo-1 ,6-dihydropyridine-2- carboxylic acid, hydrobromide salt (P1 ).
A solution of C2 (3.82 g, 15.9 mmol) in acetic acid (30 mL) and aqueous hydrobromic acid (48%, 30 mL) was heated at reflux for 4 hours. The reaction was allowed to cool to room temperature, and then chilled in an ice bath; the resulting precipitate was collected via filtration and washed with ice water (30 mL).
Recrystallization from ethanol (20 mL) provided the title compound as a light yellow solid. Yield: 3.79 g, 12.6 mmol, 79%. LCMS m/z 220.1 (M+1 ). 1H N MR (400 MHz, DMSO-c/6) δ 12.6 (v br s, 1 H), 9.58-9.60 (m, 1 H), 8.07 (d, J=7.6 Hz, 1 H), 7.88-7.91 (m, 1 H), 7.09 (d, J=7.4 Hz, 1 H), 2.34 (br s, 3H). Preparation P2: 5-(4-Methyl-1 H-imidazol-1 -yl)-6-oxo-1 ,6-dihvdropyridine-2-carboxylic acid, hydrochloride salt (P2)
A mixture of C2 (12.8 g, 51 .8 mmol) and 37% hydrochloric acid (25 mL) was heated at reflux for 18 hours. After the reaction mixture had cooled to room
temperature, the solid was collected via filtration; it was stirred with 1 ,4-dioxane (2 x 20 mL) and filtered again, to afford the product as a yellow solid. Yield: 13 g, 51 mmol, 98%. 1H NMR (400 MHz, CD3OD) δ 9.52 (br s, 1 H), 8.07 (d, J=7.5 Hz, 1 H), 7.78 (br s, 1 H), 7.21 (d, J=7.5 Hz, 1 H), 2.44 (s, 3H). Preparation P3: 7-(4-Methyl-1 H-imidazol-1 -yl)-3,4-dihvdropyridor2,1 -ciri ,41oxazine-1 ,6- dione (P3)
Compound P2 (65 g, 250 mmol), 1 ,2-dibromoethane (52.5 g, 280 mmol) and cesium carbonate (124 g, 381 mmol) were combined in A/,/V-dimethylformamide (850 mL) and heated at 90 °C for 6 hours. The reaction mixture was then cooled and filtered through Celite. After concentration of the filtrate in vacuo, the residue was dissolved in dichloromethane (500 mL), washed with saturated aqueous sodium chloride solution (100 mL), washed with water (50 mL), dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The resulting solid was washed with acetonitrile to provide the product. Yield: 46.5 g, 190 mmol, 76%. 1H NMR (400 MHz, CDCI3) δ 8.33 (d, J=1 .4 Hz, 1 H), 7.43 (AB quartet, JAB=7.7 Hz, ΔνΑΒ=33.4 Hz, 2H), 7.15-7.17 (m, 1 H), 4.66-4.70 (m, 2H), 4.38-4.42 (m, 2H), 2.30 (d, J=0.8 Hz, 3H).
//////////PF-06648671, PF 06648671, PF-6648671, PHASE 1
FC(F)(F)c5cc(Cl)c(F)cc5[C@H]1CCC[C@H](O1)[C@H](C)N4CCN3C(=CC=C(n2cc(C)nc2)C3=O)C4=O
First speaker of the PM session is Martin Pettersson from @pfizer talking about a gamma secretase modulator for Alzheimer’s #ACSSanFran