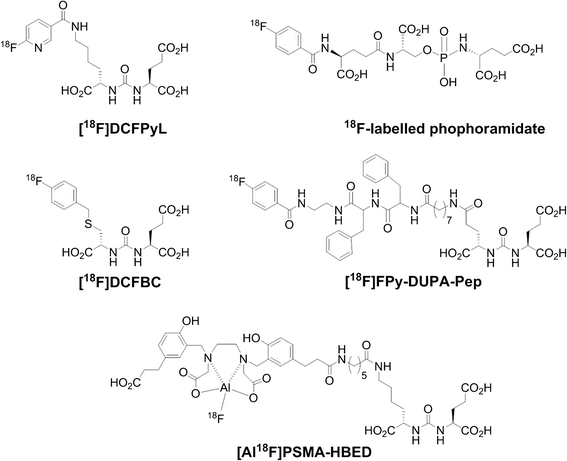
Piflufolastat F 18 injection
Dcfpyl F-18
CAS 207181-29-0
PLAIN F 1423758-00-2 WITHOUT RADIO LABEL
Other Names
- N-[[[(1S)-1-Carboxy-5-[[[6-(fluoro-18F)-3-pyridinyl]carbonyl]amino]pentyl]amino]carbonyl]-L-glutamic acid
- [18F]DCFPyl
Dcfpyl F-18
(18F)Dcfpyl
UNII-3934EF02T7
18F-DCFPyL
3934EF02T7
Progenics Pharmaceuticals, Inc.
APPROVED 5/26/2021 fda, Pylarify
For positron emission tomography imaging of prostate-specific membrane antigen-positive lesions in men with prostate cancer
For positron emission tomography (PET) of prostatespecific membrane antigen (PSMA) positive lesions in men with prostate cancer: • with suspected metastasis who are candidates for initial definitive therapy. • with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
- Originator Johns Hopkins University School of Medicine
- Developer Curium Pharma; Progenics Pharmaceuticals
- Class Amides; Carboxylic acids; Fluorinated hydrocarbons; Imaging agents; Pyridines; Radiopharmaceutical diagnostics; Radiopharmaceuticals; Small molecules; Urea compounds
- Mechanism of ActionPositron-emission tomography enhancers
- Orphan Drug StatusNo
- MarketedProstate cancer
- 28 May 2021Registered for Prostate cancer (Diagnosis) in USA (IV) – First global approval
- 28 May 2021Adverse events data from phase III CONDOR and phase II/III OSPREY trials in prostate cancer released by Lantheus Holdings
- 27 May 2021Lantheus Holdings intends to launch Fluorine-18 DCFPyL in USA at end of 2021
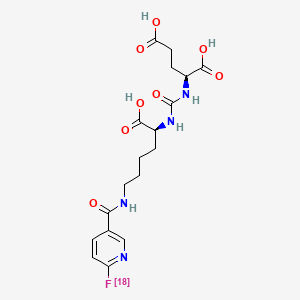
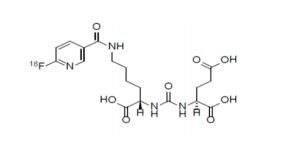
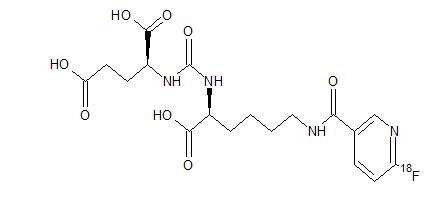
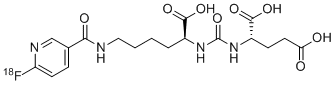
PYLARIFY contains fluorine 18 (F 18), radiolabeled prostate-specific membrane antigen inhibitor imaging agent. Chemically piflufolastat F 18 is 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl) amino]-pentyl}ureido)-pentanedioic acid. The molecular weight is 441.4 and the structural formula is:
The chiral purity of the unlabeled piflufolastat F 18 precursor is greater than 99% (S,S). PYLARIFY is a sterile, non-pyrogenic, clear, colorless solution for intravenous injection. Each milliliter contains 37 to 2,960 MBq (1 to 80 mCi) piflufolastat F 18 with ≤0.01 µg/mCi of piflufolastat at calibration time and date, and ≤ 78.9 mg ethanol in 0.9% sodium chloride injection USP. The pH of the solution is 4.5 to 7.0. PYLARIFY has a radiochemical purity of at least 95% up to 10 hours following end of synthesis, and specific activity of at least 1000 mCi/µmol at the time of administration.
PYLARIFY contains fluorine 18 (F 18), radiolabeled prostate-specific membrane antigen inhibitor imaging agent. Chemically piflufolastat F 18 is 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)amino]-pentyl}ureido)-pentanedioic acid. The molecular weight is 441.4 and the structural formula is:
 |
The chiral purity of the unlabeled piflufolastat F 18 precursor is greater than 99% (S,S).
PYLARIFY is a sterile, non-pyrogenic, clear, colorless solution for intravenous injection. Each milliliter contains 37 to 2,960 MBq (1 to 80 mCi) piflufolastat F 18 with ≤0.01 μg/mCi of piflufolastat at calibration time and date, and ≤ 78.9 mg ethanol in 0.9% sodium chloride injection USP. The pH of the solution is 4.5 to 7.0.
PYLARIFY has a radiochemical purity of at least 95% up to 10 hours following end of synthesis, and specific activity of at least 1000 mCi/μmol at the time of administration.
Physical Characteristics
PYLARIFY is radiolabeled with fluorine 18 (F 18), a cyclotron produced radionuclide that decays by positron emission to stable oxygen 18 with a half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 3).
Table 3: Principal Radiation Produced from Decay of Fluorine 18
| Radiation Energy (keV) | Abundance (%) | |
| Positron | 249.8 | 96.9 |
| Gamma | 511 | 193.5 |
FDA
- Approval Letter(s) (PDF)
- Printed Labeling (PDF)
- Product Quality Review(s) (PDF)
- Multi-Discipline Review (PDF)
- Proprietary Name Review(s) (PDF)
- Officer/Employee List (PDF)
- Other Review(s) (PDF)
- Risk Assessment and Risk Mitigation Review(s) (PDF)
- Administrative and Correspondence Documents (PDF)
PATENT
WO 2016030329
WO 2017072200
PAPER
Journal of Labelled Compounds and Radiopharmaceuticals (2016), 59(11), 439-450
CLIP
https://ejnmmires.springeropen.com/articles/10.1186/s13550-016-0195-6
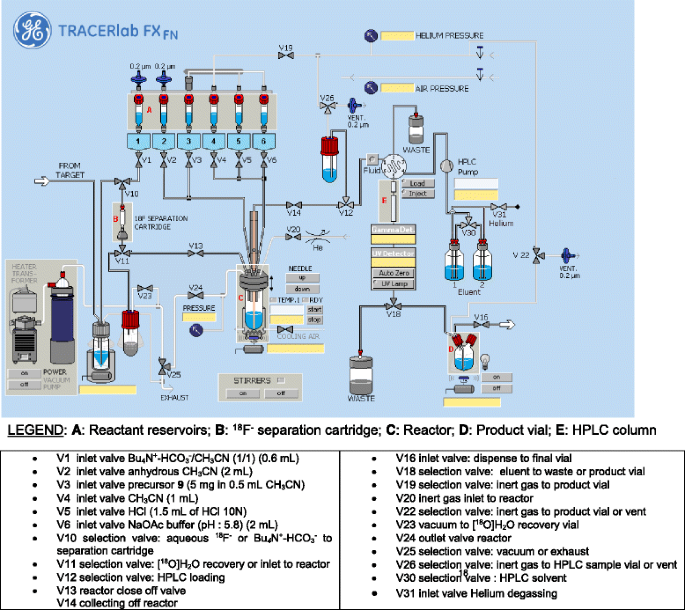
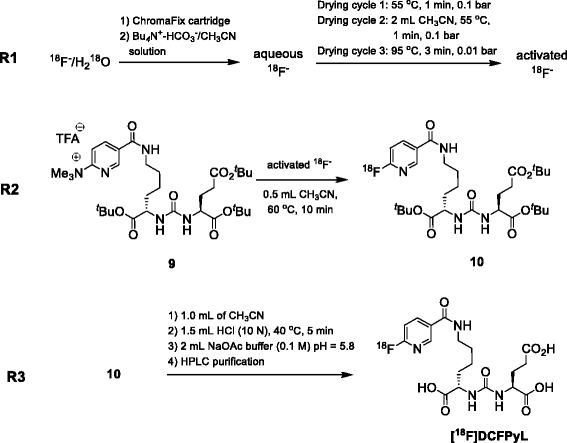
![Radiosynthesis of [ 18 F]DCFPyL](https://www.researchgate.net/publication/301830909/figure/fig4/AS:362696278069251@1463484939571/Radiosynthesis-of-18-FDCFPyL.png)
/////////piflufolastat F 18, injection, Orphan Drug , Prostate cancer, [18F]DCFPyL, 18F-DCFPYL, DCFPYL F-18, fda 2021, approvals 2021
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214793Orig1s000ChemR.pdf
- Product Quality Review(s) (PDF)
- Multi-Discipline Review (PDF)
- Proprietary Name Review(s) (PDF)
- Officer/Employee List (PDF)
- Other Review(s) (PDF)
- Risk Assessment and Risk Mitigation Review(s) (PDF)
- Administrative and Correspondence Documents (PDF)
- Label (PDF)
C1=CC(=NC=C1C(=O)NCCCCC(C(=O)O)NC(=O)NC(CCC(=O)O)C(=O)O)F