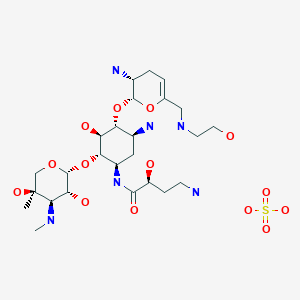
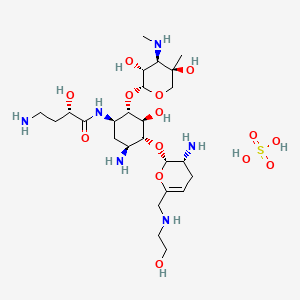
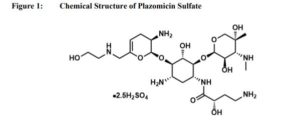
Plazomicin
- Molecular FormulaC25H48N6O10
- Average mass592.683 Da
Plazomicin Sulfate
| Molecular Formula: | C25H50N6O14S |
|---|---|
| Molecular Weight: | 690.763 g/mol |
Plazomicin Sulfate; UNII-A78L6MT746; Plazomicin Sulfate [USAN]; A78L6MT746; 1380078-95-4; Plazomicin sulfate (USAN),
|
6′-(hydroxylethyl)-1-(haba)-sisomicin
Plazomicin is a neoglycoside antibiotic with activity against a broad range of Gram-positive and Gram-negive pathogens. Plazomicin showed potent in vitro activity against multidrug-resistant Klebsiella pneumoniae and Escherichia coli.
- Mechanism of ActionProtein synthesis inhibitors
- Orphan Drug StatusNo
- New Molecular EntityYes
Highest Development Phases
- MarketedUrinary tract infections
- RegisteredPyelonephritis
- PreregistrationBacteraemia; Nosocomial pneumonia
- PreclinicalGram-negative infections
- No development reportedRespiratory tract infections; Tularaemia; Yersinia infections
Most Recent Events
- 27 Jun 2018Registered for Pyelonephritis (Treatment-resistant) in USA (IV)- First Global Approval
- 27 Jun 2018Registered for Urinary tract infections (Treatment-resistant) in USA (IV)- First Global Approval
- 26 Jun 2018Achaogen receives complete response letter from the FDA for Plazomicin in Bloodstream infection
| Synonyms: O-2-Amino-2,3,4,6-tetradeoxy-6-[(2-hydroxyethyl)amino]-α-D-glycero-hex-4-enopyranosyl-(1→4)-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl-(1→6)]-N1-[(2S)-4-amino-2-hydroxy-1-oxobutyl]-2-deoxy-D-streptamine; ACHN 490; |
| CAS Number: 1154757-24-0
Sulfate 1380078-95-4, プラゾマイシン硫酸塩; |
| Achaogen (USA)Phase II completed |
| Mol. Formula: C25H48N6O10 |
| Aminoglycosides, Broad-spectrum, |
| Mol. Weight: 592.68 |
FDA
https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf
Developed by Achaogen biopharmaceuticals, plazomicin is a next-generation aminoglycoside synthetically derived from [DB12604]. The structure of plazomicin was established via appending hydroxylaminobutyric acid to [DB12604] at position 1 and 2-hydroxyethyl group at position 6′ [A33942]. It was designed to evade all clinically relevant aminoglycoside-modifying enzymes, which contribute to the main resistance mechanism for aminoglycoside therapy [A33942]. However, acquired resistance of aminoglycosides may arise through over expression of efflux pumps and ribosomal modification by bacteria, which results from amino acid or rRNA sequence mutations [A33942]. Like other aminoglycosides, plazomicin is ineffective against bacterial isolates that produce 16S rRNA methyltransferases [FDA Label]. Plazomicin mediates the antibacterial activity against pathogens including carbapenem-resistant (CRE) and extended-spectrum beta-lactamase (ESBL) producing _Enterobacteriaceae_. It mediates the antibacterial activity by binding to bacterial 30S ribosomal subunit and inhibiting protein synthesis [FDA Label]. On June 28th, 2018, plazomicin sulfate was approved by the FDA for use in adult patients for the treatment of complicated urinary tract infections (cUTI) including Pyelonephritis. It is marketed as Zemdri and is administered via once-daily intravenous infusion.
Developed by Achaogen biopharmaceuticals, plazomicin is a next-generation aminoglycoside synthetically derived from Sisomicin. The structure of plazomicin was established via appending hydroxylaminobutyric acid to Sisomicin at position 1 and 2-hydroxyethyl group at position 6′ [1]. It was designed to evade all clinically relevant aminoglycoside-modifying enzymes, which contribute to the main resistance mechanism for aminoglycoside therapy [1]. However, acquired resistance of aminoglycosides may arise through over expression of efflux pumps and ribosomal modification by bacteria, which results from amino acid or rRNA sequence mutations [1]. Like other aminoglycosides, plazomicin is ineffective against bacterial isolates that produce 16S rRNA methyltransferases [Label]. Plazomicin mediates the antibacterial activity against pathogens including carbapenem-resistant (CRE) and extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae. It mediates the antibacterial activity by binding to bacterial 30S ribosomal subunit and inhibiting protein synthesis [Label]. On June 28th, 2018, plazomicin sulfate was approved by the FDA for use in adult patients for the treatment of complicated urinary tract infections (cUTI) including Pyelonephritis. It is marketed as Zemdri and is administered via once-daily intravenous infusion.
Plazomicin (INN,[1] ZEMDRI) is a next-generation aminoglycoside (“neoglycoside”) antibacterial derived from sisomicin by appending a hydroxy-aminobutyric acid (HABA) substituent at position 1 and a hydroxyethyl substituent at position 6′.[2][3]
Plazomicin has been reported to demonstrate in vitro synergistic activity when combined with daptomycin or ceftobiprole versus methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA) and against Pseudomonas aeruginosawhen combined with cefepime, doripenem, imipenem or piperacillin/tazobactam.[3] It also demonstrates potent in vitro activity versus carbapenem-resistant Acinetobacter baumannii.[4]
In 2012, U.S. Food and Drug Administration granted fast track designation for the development and regulatory review of plazomicin.[5]
It is being developed by Achaogen, Inc. to treat serious bacterial infections due to multidrug-resistant Enterobacteriaceae, including carbapenem-resistant Enterobacteriaceae (CRE)[6] and was in Phase III clinical trials as of April 7, 2016.[7]
In June 2018 the FDA approved plazomicin (ZEMDRI) for adults with complicated urinary tract infections (cUTI), including pyelonephritis, caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Enterobacter cloacae, in patients who have limited or no alternative treatment options. Zemdri is an intravenous infusion, administered once daily.[8][9] The FDA declined approval for treating bloodstream infections due to lack of effectiveness.[10]
To continue the development of plazomicin, the company has received a contract option of US$ 60M from the Biomedical Advanced Research and Development Authority (BARDA) to support a global Phase III clinical study. The study will evaluate plazomicin in treating patients with serious Gram-negative bacterial infections due to carbapenem-resistant Enterobacteriaceae. The study is expected to start in the fourth quarter of 2013 [4].
PATENT
WO 2009067692
WO 2010132770
PAPER
Synthesis and spectrum of the neoglycoside ACHN-490
Antimicrobial Agents and Chemotherapy (2010), 54, (11), 4636-4642
https://aac.asm.org/content/54/11/4636



PAPER
Plazomicin Retains Antibiotic Activity against Most Aminoglycoside Modifying Enzymes
ACS Infectious Diseases (2018), 4, (6), 980-987.
https://pubs.acs.org/doi/abs/10.1021/acsinfecdis.8b00001
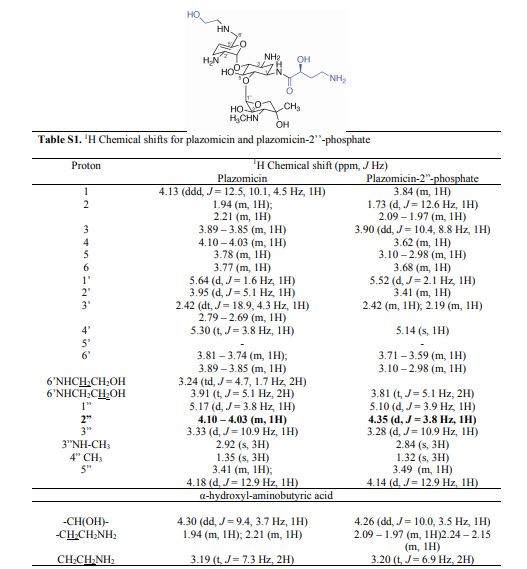
PAPER
Effects of the 1-N-(4-Amino-2S-hydroxybutyryl) and 6′-N-(2-Hydroxyethyl) Substituents on Ribosomal Selectivity, Cochleotoxicity, and Antibacterial Activity in the Sisomicin Class of Aminoglycoside Antibiotics
ACS Infectious Diseases (2018), 4, (7), 1114-1120.
https://pubs.acs.org/doi/abs/10.1021/acsinfecdis.8b00052

Syntheses of the 6′-N-(2-hydroxyethyl) and 1-N-(4-amino-2S-hydroxybutyryl) derivatives of the 4,6-aminoglycoside sisomicin and that of the doubly modified 1-N-(4-amino-2S-hydroxybutyryl)-6′-N-(2-hydroxyethyl) derivative known as plazomicin are reported together with their antibacterial and antiribosomal activities and selectivities. The 6′-N-(2-hydroxyethyl) modification results in a moderate increase in prokaryotic/eukaryotic ribosomal selectivity, whereas the 1-N-(4-amino-2S-hydroxybutyryl) modification has the opposite effect. When combined in plazomicin, the effects of the two groups on ribosomal selectivity cancel each other out, leading to the prediction that plazomicin will exhibit ototoxicity comparable to those of the parent and the current clinical aminoglycoside antibiotics gentamicin and tobramycin, as borne out by ex vivo studies with mouse cochlear explants. The 6′-N-(2-hydroxyethyl) modification restores antibacterial activity in the presence of the AAC(6′) aminoglycoside-modifying enzymes, while the 1-N-(4-amino-2S-hydroxybutyryl) modification overcomes resistance to the AAC(2′) class but is still affected to some extent by the AAC(3) class. Neither modification is able to circumvent the ArmA ribosomal methyltransferase-induced aminoglycoside resistance. The use of phenyltriazenyl protection for the secondary amino group of sisomicin facilitates the synthesis of each derivative and their characterization through the provision of sharp NMR spectra for all intermediates.
https://pubs.acs.org/doi/suppl/10.1021/acsinfecdis.8b00052/suppl_file/id8b00052_si_001.pdf
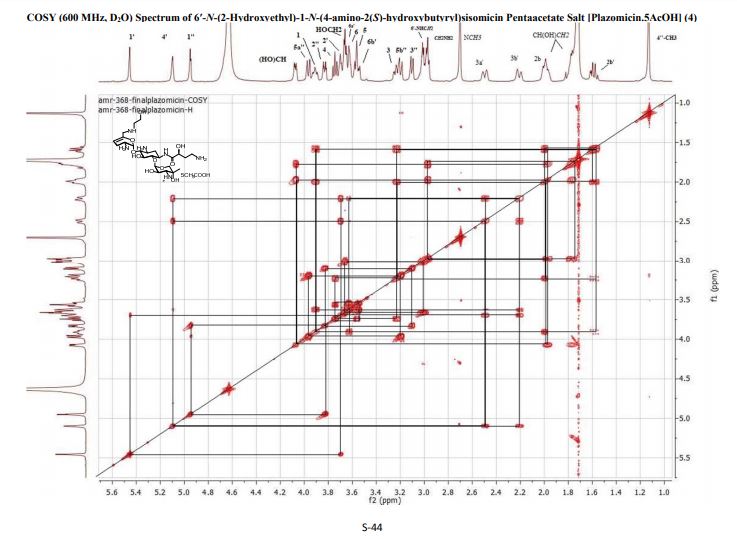
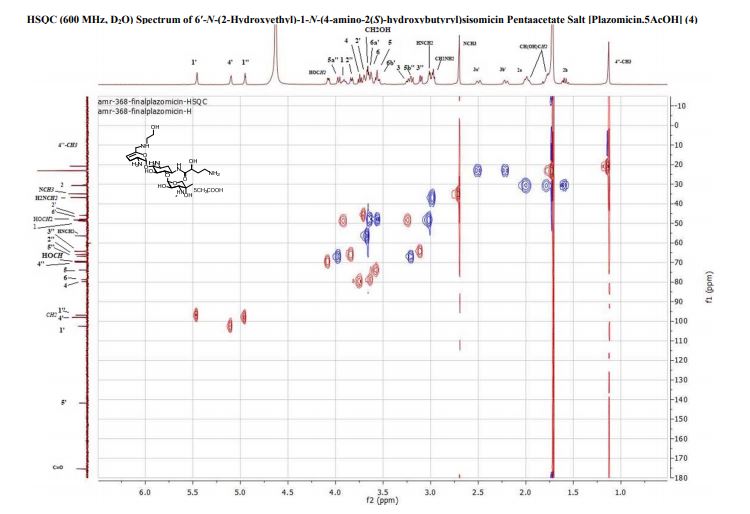
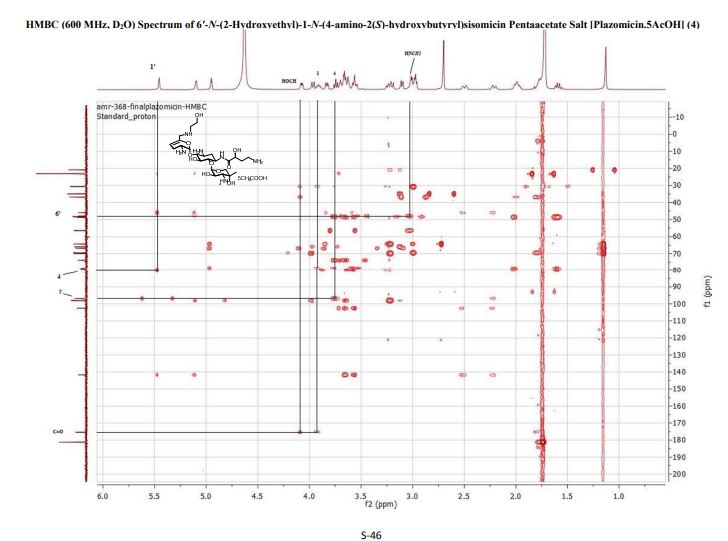
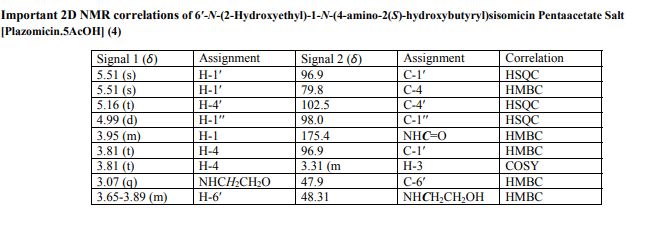
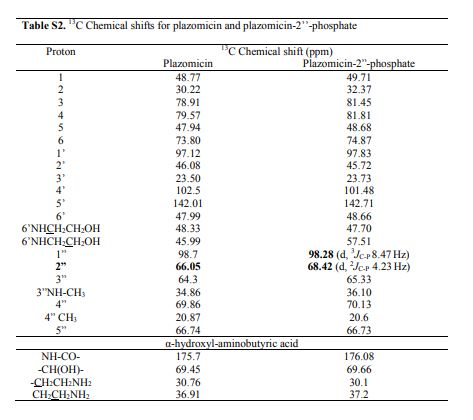
4 (19 mg, 40%). [α]D 25 = +46.5 (c = 0.01, H2O);
1 H NMR (600 MHz, D2O): δ 5.51 ( s, 1H, H-1ʹ), 5.16 (t, J = 3.5 Hz, H, H-4ʹ), 4.99 (d , J = 4.0 Hz, 1H, H-1ʹʹ), 4.11 (dd , J =9.4 Hz, 3.9 Hz, 1H, CH(OH)CH2CH2), 4.00 (d , J = 12.8 Hz, 1H, H-5ʹʹ), 3.99-3.93 (m, 1H, H-1), 3.84 (dd, J = 11.0 Hz, 4.0 Hz, 1H, H-2ʹʹ), 3.81 (t, J = 9.9 Hz, 1H, H-4), 3.77 (t, J = 5.3 Hz, 1H, H-2ʹ), 3.71 (t, J = 5.1 Hz, 2H, NHCH2CH2O), 3.69 – 3.65 (m, 2H, H-6, H-6ʹ), 3.64 – 3.44 (m , 2H, H-5, H-6ʹ), 3.35 – 3.26 (m , 1H, H-3), 3.24 (d, J = 12.8 Hz, 1H, H-5ʹʹ), 3.15 (d, J = 11.0 Hz, 1H, H-3ʹʹ), 3.09 – 3.06 (m, 2H, NHCH2CH2O), 3.01 (t, J = 7.2 Hz, 2H, CH(OH)CH2CH2), 2.74 (s, 3H, NCH3), 2.58 – 2.52 (m, 1H, H-3ʹ), 2.29 – 2.24 (m, 1H, H-3ʹ), 2.07 (dt, J = 13.2 Hz, 4.4 Hz, 1H, H-2), 2.04 – 1.98 (m, 1H, CH(OH)CH2CH2), 1.84 – 1.79 (m, 1H, CH(OH)CH2CH2), 1.64 (q, 1H, J = 12.5 Hz, H-2), 1.17 (s, 3H, 4ʹʹ-CH3);
13C NMR (151 MHz, D2O): δ 181.2 (s, CH3COOH), 175.4 (s, NHCO), 141.7 (s, C-5ʹ), 102.5 (s, C-4ʹ), 98.0 (s, C-1ʹʹ), 96.9 (s, C-1ʹ), 79.8 (s, C-4), 78.8 (s, C-6), 73.8 (s, C-5), 69.8 (s, C-4ʹʹ), 69.4 (s, CH(OH)CH2CH2), 66.8 (s, C-5ʹʹ), 65.9 (s, C-2ʹʹ), 64.2 (s, C-3ʹʹ), 56.4 (s, NHCH2CH2O), 48.8 (s, C-1), 48.31 (s, NHCH2CH2O), 48.26 (s, C-3), 47.9 (s, C-6ʹ), 45.9 (s, C2ʹ), 36.8 (s, CH(OH)CH2CH2), 34.9 (s, NCH3), 30.7 (s, CH(OH)CH2CH2), 30.4 (s, C-2), 23.1 (s, CH3COOH), 23.0 (s, C-3ʹ), 20.8 (s, 4ʹʹ-CH3).
ESI-HRMS: m/z calcd. for C25H49N6O10 [M+H]+ 593.3510, found: 593.3481.
PATENT
http://www.google.com/patents/US20100099661
Common Intermediates Sisomicin
Amberlite IRA-400 (OH form) (200 g) was washed with MeOH (3×200 m1). To a stirring suspension of the washed resin in MeOH (150 mL) was added sisomicin sulfate (20.0 g, 0.029 mol) and the mixture was stirred overnight. The resin was then filtered and washed with MeOH (100 mL) and the combined organic layers were concentrated to dryness to yield the desired sisomicin (11.57 g, 0.026 mol, 89.6% yield): MS m/e [M+H]+ calcd 448.3, found 448.1.
Example 1 6′-(2-Hydroxy-ethyl)-1-(4-amino-2(S)-hydroxy-butyryl)-sisomicin
6′-(2-tert-Butyldimethylsililoxy-ethyl)-2′,3,3″-triBoc-1-(N-Boc-4-amino-2(S)-hydroxy-butyryl)-sisomicin
2′,3,3″-triBoc-1-(N-Boc-4-amino-2(S)-hydroxy-butyryl)-sisomicin (0.10 g, 0.105 mmol) was treated with tert-butyldimethylsilyloxy acetaldehyde following Procedure 1-Method A to yield the desired 6′-(2-tert-butyldimethylsilyloxy-ethyl)-2′,3,3″-triBoc-1-(N-Boc-4-amino-2(S)-hydroxy-butyryl)-sisomicin (MS m/e [M+H]+ calcd 1107.6, found 1107.4), which was carried through to the next step without further purification.
6′-(2-Hydroxy-ethyl)-1-(4-amino-2(S)-hydroxy-butyryl)-sisomicin
6′ -(2-tert-butyldimethylsililoxy-ethyl)-2′,3,3″-triBoc-1-(N-Boc-4-amino-2(S)-hydroxy-butyryl)-sisomicin (0.105 mmol) was submitted to Procedure 3-Method B for Boc removal to yield a crude, which was purified by RP HPLC Method 1-Column A to yield 6′-(2-hydroxy-ethyl)-1-(4-amino-2(S)-hydroxy-butyryl)-sisomicin: MS m/e [M+H]+ calcd 593.3, found 593.2, [M+Na]+615.3 ; CLND 97.5% purity.
- Achaogen. Study for the treatment of complicated urinary tract infection and acute pyelonephritis.Available online: http://www.clinicaltrials.gov/ct2/show/NCT01096849 (accessed on 11 April 2013).
- Zhanel, G.G.; Lawson, C.D.; Zelenitsky, S.; Findlay, B.; Schweizer, F.; Adam, H.; Walkty, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev. Anti-Infect. Ther. 2012, 10, 459–473, doi:10.1586/eri.12.25.
- Endimiani, A.; Hujer, K.M.; Hujer, A.M.; Armstrong, E.S.; Choudhary, Y.; Aggen, J.B.; Bonomo, R.A. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2009, 53, 4504–4507.
- Achaogen. Achaogen pipeline. Available online: http://www.achaogen.com (accessed on 30 August 2012).
- Achaogen. Achaogen Awarded $60M Contract Option by BARDA for the Clinical Development of Plazomicin. Available online: http://www.achaogen.com/news/151/15 (accessed on 19 June 2013).
- Achaogen. Achaogen announces all objectives met in Phase 2 Plazomicin complicated urinary tract infections study and start of first-in-human study with ACHN-975. Available online: http://www.achaogen.com/uploads/news/id148/Achaogen_PressRelease_2012–05–15.pdf (accessed on 10 April 2013).
- Achaogen. Achaogen Announces Agreement with FDA on a Special Protocol Assessment for a Phase 3 Clinical Trial of Plazomicin to Treat Infections Caused by Carbapenem-Resistant Enterobacteriaceae (CRE); Achaogen: San Francisco, CA, USA, 2013.
- Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin
-
4-23-2010ANTIBACTERIAL AMINOGLYCOSIDE ANALOGS

| US8318685 | Nov 14, 2011 | Nov 27, 2012 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8367625 | Apr 7, 2011 | Feb 5, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8372813 | Apr 7, 2011 | Feb 12, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8377896 | Mar 9, 2011 | Feb 19, 2013 | Isis Pharmaceuticals, Inc | Antibacterial 4,6-substituted 6′, 6″ and 1 modified aminoglycoside analogs |
| US8399419 | Mar 9, 2011 | Mar 19, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8481502 | Apr 6, 2012 | Jul 9, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8492354 | Nov 14, 2011 | Jul 23, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8524675 | Nov 14, 2011 | Sep 3, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8524689 | Nov 14, 2011 | Sep 3, 2013 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8569264 | Jan 5, 2012 | Oct 29, 2013 | Isis Pharmaceuticals, Inc. | Antibacterial 4,5-substituted aminoglycoside analogs having multiple substituents |
| US8653041 | Oct 15, 2012 | Feb 18, 2014 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8653042 | Nov 14, 2011 | Feb 18, 2014 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
| US8658606 | Nov 14, 2011 | Feb 25, 2014 | Achaogen, Inc. | Antibacterial aminoglycoside analogs |
References
- Jump up^ “WHO Drug Information, Vol. 26, No. 3, 2012. International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 68”(PDF). World Health Organization. p. 314. Retrieved 27 April 2016.
- Jump up^ Aggen, JB; Armstrong, ES; Goldblum, AA; Dozzo, P; Linsell, MS; Gliedt, MJ; Hildebrandt, DJ; Feeney, LA; Kubo, A; Matias, RD; Lopez, S; Gomez, M; Wlasichuk, KB; Diokno, R; Miller, GH; Moser, HE (30 August 2010). “Synthesis and Spectrum of the Neoglycoside ACHN-490” (PDF). Antimicrobial Agents and Chemotherapy. 54 (11): 4636–4642. doi:10.1128/AAC.00572-10. PMC 2976124
 . PMID 20805391. Retrieved 27 April2016.
. PMID 20805391. Retrieved 27 April2016. - ^ Jump up to:a b Zhanel, GG; Lawson, CD; Zelenitsky, S; Findlay, B; Schweizer, F; Adam, H; Walkty, A; Rubinstein, E; Gin, AS; Hoban, DJ; Lynch, JP; Karlowsky, JA (10 January 2014). “Comparison of the Next-Generation Aminoglycoside Plazomicin to Gentamicin, Tobramycin and Amikacin”. Expert Review of Anti-infective Therapy. 10 (4): 459–73. doi:10.1586/eri.12.25. PMID 22512755.
- Jump up^ García-Salguero, C; Rodríguez-Avial, I; Picazo, JJ; Culebras, E (October 2015). “Can Plazomicin Alone or in Combination Be a Therapeutic Option against Carbapenem-Resistant Acinetobacter baumannii?” (PDF). Antimicrobial Agents and Chemotherapy. 59 (10): 5959–66. doi:10.1128/AAC.00873-15. PMC 4576036
 . Retrieved 27 April 2016.
. Retrieved 27 April 2016. - Jump up^ “Achaogen Announces Plazomicin Granted QIDP Designation by FDA”. GlobeNewswire, Inc. Retrieved 27 April 2016.
- Jump up^ “Achaogen — Plazomicin”. Achaogen, Inc. Retrieved 27 April2016.
- Jump up^ “Plazomicin — AdisInsight”. Springer International Publishing AG. Retrieved 27 April 2016.
- Jump up^ “Medscape Log In”. www.medscape.com. Retrieved 2018-07-03.
- Jump up^ “BioCentury – FDA approves plazomicin for cUTI, but not blood infections”. www.biocentury.com. Retrieved 2018-06-28.
- Jump up^ “Drugs@FDA: FDA Approved Drug Products”. www.accessdata.fda.gov. Retrieved 2018-06-28.
 |
|
| Names | |
|---|---|
| IUPAC name
(2S)-4-Amino-N-[(1R,2S,3S,4R,5S)-5-amino-4-[[(2S,3R)-3-amino-6-[(2-hydroxyethylamino)methyl]-3,4-dihydro-2H-pyran-2-yl]oxy]-2-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-(methylamino)oxan-2-yl]oxy-3-hydroxycyclohexyl]-2-hydroxybutanamide
|
|
| Other names
6′-(hydroxylethyl)-1-(HABA)-sisomicin
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| C25H48N6O | |
| Molar mass | 592.683 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F],
|
|
Achaogen is a clinical-stage biopharmaceutical company passionately committed to the discovery, development, and commercialization of novel antibacterials to treat multi-drug resistant, or MDR, gram-negative infections.

Achaogen (a-KAY-o-jen) is developing plazomicin, its lead product candidate, for the treatment of serious bacterial infections due to MDR Enterobacteriaceae, including carbapenem-resistant Enterobacteriaceae, or CRE. In 2013, the Centers for Disease Control and Prevention identified CRE as a “nightmare bacteria” and an immediate public health threat that requires “urgent and aggressive action.” We expect to initiate a Phase 3 superiority trial of plazomicin in the first quarter of 2014.
CRE are one of many types of MDR gram-negative pathogens threatening patients. Bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii, and extended-spectrum beta-lactamase producing Enterobacteriaceae each pose “serious” resistance threats, according to the CDC, and also drive a great need for new, safe, and effective antibiotics. We have assembled the chemistry and microbiology expertise and capabilities required to develop new agents for the treatment of gram-negative infections. Plazomicin was the first clinical candidate from our gram-negative antibiotic discovery engine. In addition, our research and development pipeline includes two antipseudomonal programs targeting P. aeruginosa—a program to discover and develop small molecule inhibitors of LpxC, which is an enzyme essential for the synthesis of the outer membrane of gram-negative bacteria, and a therapeutic antibody program. We are also pursuing small molecule research programs targeting other essential gram-negative enzymes.
Achaogen has built an exceptional research and development team with deep expertise in the discovery and development of new drugs from research through commercialization. Our executive team has over 60 years of combined industry experience, and a proven track record of leadership, global registration, and lifecycle management for over 20 products. Our facility is located on the shores of the San Francisco Bay, ten minutes from the San Francisco International Airport, and only fifteen minutes from downtown San Francisco.

ZEMDRITM (plazomicin) Approved by FDA for the Treatment of Adults with Complicated Urinary Tract Infections (cUTI)
― ZEMDRI is a new treatment for patients with cUTI, including pyelonephritis, due to certain Enterobacteriaceae ―
― ZEMDRI is the only once-daily aminoglycoside therapy approved for use in cUTI ―
― ZEMDRI has microbiological activity against pathogens designated by the CDC as urgent and serious public health threats, including carbapenem-resistant (CRE) and extended spectrum beta-lactamase (ESBL)- producing Enterobacteriaceae ―
SOUTH SAN FRANCISCO, Calif., June 26, 2018 (GLOBE NEWSWIRE) — Achaogen, Inc. (NASDAQ:AKAO), a biopharmaceutical company developing and commercializing innovative antibacterial agents to address multidrug resistant (MDR) gram-negative infections, today announced that the U.S. Food and Drug Administration (FDA) has approved ZEMDRI™ (plazomicin) for adults with complicated urinary tract infections (cUTI), including pyelonephritis, caused by certain Enterobacteriaceae in patients who have limited or no alternative treatment options. ZEMDRI is an intravenous infusion, administered once daily.
“The approval of ZEMDRI marks a significant milestone for Achaogen and we are excited to offer healthcare practitioners a new treatment option for patients with certain serious bacterial infections. ZEMDRI is designed to retain its potent activity in the face of certain difficult-to-treat MDR infections, including CRE and ESBL- producing Enterobacteriaceae,” said Blake Wise, Achaogen’s Chief Executive Officer. “Today’s milestone was made possible by our employees, by patients and investigators involved in our clinical trials, and by BARDA, who contributed significant funding for the development of ZEMDRI. This marks an important step in our commitment to fighting MDR bacteria and we are excited to launch ZEMDRI, a much needed once-daily antibiotic.”
“Bacteria continue to circumvent existing antibiotics, making certain infections notoriously hard to treat and putting some patients at high risk for mortality,” said James A. McKinnell, Assistant Professor of Medicine at the David Geffen School of Medicine and LA Biomed at Harbor-UCLA. “Aminoglycosides are a familiar and very effective class of antibiotics. I look forward to adding plazomicin to my short list of available treatment options and to its potential impact on patient outcomes.”
Regarding the potential indication for plazomicin for the treatment of bloodstream infection (BSI), the FDA issued a Complete Response Letter (CRL) stating that the CARE study does not provide substantial evidence of effectiveness of plazomicin for the treatment of BSI. The Company intends to meet with the FDA to determine whether there is a feasible resolution to address the CRL.
Achaogen will work with hospitals, providers, and insurers to ensure patients are able to receive this treatment. Patients, physicians, pharmacists, or other healthcare professionals with questions about ZEMDRI should contact 1.833.252.6400 or visit www.ZEMDRI.com.
ZEMDRI Phase 3 Clinical Results
The approval of ZEMDRI is supported in part by data from the EPIC (Evaluating Plazomicin In cUTI) clinical trial, which was the first randomized controlled study of once-daily aminoglycoside therapy for the treatment of cUTI, including pyelonephritis.
In the Phase 3 EPIC cUTI trial, ZEMDRI demonstrated non-inferiority to meropenem for the co-primary efficacy endpoints of composite cure (clinical cure and microbiological eradication) in the microbiological modified intent-to-treat (mMITT; N=388) population at Day 5 and test-of-cure (TOC) visit (Day 17 + 2). Composite cure rates at Day 5 were 88.0% (168/191) for ZEMDRI vs 91.4% (180/197) for meropenem (difference -3.4%, 95% CI, -10.0 to 3.1). Composite cure rates at TOC were 81.7% (156/191) for ZEMDRI vs 70.1% (138/197) for meropenem (difference 11.6%, 95% CI, 2.7 to 20.3). Composite cure at the TOC visit in patients with concomitant bacteremia at baseline was achieved in 72.0% (18/25) of patients in the ZEMDRI group and 56.5% (13/23) of patients in the meropenem group. The most common side effects (≥1% of patients treated with ZEMDRI) were decreased kidney function, diarrhea, hypertension, headache, nausea, vomiting, and hypotension.1
The FDA approved a breakpoint of <= 2 mcg/mL; greater than 99% of Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae in U.S. surveillance are susceptible to Zemdri when applying this breakpoint.2
About cUTI
cUTI is defined as a UTI occurring in a patient with an underlying complicating factor of the genitourinary tract, such as a structural or functional abnormality.3 Patients with pyelonephritis, regardless of underlying abnormalities of the urinary tract, are considered a subset of patients with cUTI.4 An estimated 3 million cases of cUTI are treated in the hospital setting in the US each year.5 Enterobacteriaceae are the most common pathogens causing cUTIs6, and resistance within this family is a global concern. High rates of resistance to previous mainstays of therapy necessitate alternative treatment options. Ineffectively managed cUTI can lead to increased treatment failure rates, recurrence of infection, increased re-hospitalization, and increased morbidity and mortality. cUTI infections place an economic burden on hospitals and payers.6,7
About ZEMDRI
ZEMDRI is an aminoglycoside with once-daily dosing that has activity against certain Enterobacteriaceae, including CRE and ESBL- producing Enterobacteriaceae. Achaogen’s EPIC clinical trial successfully evaluated the safety and efficacy of ZEMDRI in adult patients with cUTI, including pyelonephritis. ZEMDRI was engineered to overcome aminoglycoside-modifying enzymes, the most common aminoglycoside-resistance mechanism in Enterobacteriaceae, and has in vitro activity against ESBL- producing, aminoglycoside- resistant, and carbapenem- resistant isolates. The Centers for Disease Control and Prevention (CDC) has characterized ESBL- producing Enterobacteriaceae as a “serious threat” and CRE as “nightmare bacteria”, which is an immediate public health threat that requires urgent and aggressive action.
Working in the Lab
Achaogen, Inc.
Blake Wise, Chief Executive Officer at Achaogen
Achaogen, Inc.
High-Resolution Achaogen company logo
Achaogen, Inc.
/////////Plazomicin, ZEMDRI, FDA 2018, fast track designation, Plazomicin SULFATE, ACHN 490 sulfate, cUTI, Achaogen
CC1(COC(C(C1NC)O)OC2C(CC(C(C2O)OC3C(CC=C(O3)CNCCO)N)N)NC(=O)C(CCN)O)O
CN[C@@H]1[C@@H](O)[C@@H](O[C@H]2[C@@H](C[C@H](N)[C@@H](O[C@H]3OC(CNCCO)=CC[C@H]3N)[C@@H]2O)NC(=O)[C@@H](O)CCN)OC[C@]1(C)O