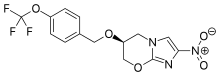
Pretomanid
プレトマニド;
| Formula |
C14H12F3N3O5
|
|---|---|
| CAS |
187235-37-6
|
| Mol weight |
359.2574
|
- (S)-PA 824
2019/8/14 FDA 2109 APPROVED
Antibacterial (tuberculostatic),
MP 149-150 °C, Li, Xiaojin; Bioorganic & Medicinal Chemistry Letters 2008, Vol 18(7), Pg 2256-2262 and Orita, Akihiro; Advanced Synthesis & Catalysis 2007, Vol 349(13), Pg 2136-2144
150-151 °C Marsini, Maurice A.; Journal of Organic Chemistry 2010, Vol 75(21), Pg 7479-7482
Pretomanid is an antibiotic used for the treatment of multi-drug-resistant tuberculosis affecting the lungs.[1] It is generally used together with bedaquiline and linezolid.[1] It is taken by mouth.[1]
The most common side effects include nerve damage, acne, vomiting, headache, low blood sugar, diarrhea, and liver inflammation.[1] It is in the nitroimidazole class of medications.[2]
Pretomanid was approved for medical use in the United States in 2019.[3][1] Pretomanid was developed by TB Alliance,[4] a not-for-profitproduct development partnership dedicated to the discovery and development of new, faster-acting and affordable medicines for tuberculosis (TB).[5]
Global Alliance for the treatment of tuberculosis (TB).
The compound was originally developed by PathoGenesis (acquired by Chiron in 2000). In 2002, a co-development agreement took place between Chiron (acquired by Novartis in 2005) and the TB Alliance for the development of the compound. The compound was licensed to Fosunpharma by TB Alliance in China.
History
Pretomanid is the generic, nonproprietary name for the novel anti-bacterial drug compound formerly called PA-824.[6] Pretomanid is referred to as “Pa” in regimen abbreviations, such as BPaL. The “preto” prefix of the compound’s name honors Pretoria, South Africa, the home of a TB Alliance clinical development office where much of the drug’s development took place. The “manid” suffix is used to group compounds with similar chemical structures. This class of drug is variously referred to as nitroimidazoles, nitroimidazooxazines or nitroimidazopyrans. Development of this compound was initiated because of the urgent need for new antibacterial drugs effective against resistant strains of tuberculosis. Also, current anti-TB drugs are mainly effective against replicating and metabolically active bacteria, creating a need for drugs effective against persisting or latent bacterial infections as often occur in patients with tuberculosis.[7]
Discovery and pre-clinical development
Pretomanid was first identified in a series of 100 nitroimidazopyran derivatives synthesized and tested for antitubercular activity. Importantly, pretomanid has activity against static M. tuberculosis isolates that survive under anaerobic conditions, with bactericidal activity comparable to that of the existing drug metronidazole. Pretomanid requires metabolic activation by Mycobacterium for antibacterial activity. Pretomanid was not the most potent compound in the series against cultures of M. tuberculosis, but it was the most active in infected mice after oral administration. Oral pretomanid was active against tuberculosis in mice and guinea pigs at safely tolerated dosages for up to 28 days.[7]
Limited FDA approval
FDA approved pretomanid only in combination with bedaquiline and linezolid for treatment of a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug resistant pulmonary tuberculosis. Pretomanid was approved under the Limited Population Pathway (LPAD pathway) for antibacterial and antifungal drugs. The LPAD Pathway was established by Congress under the 21st Century Cures Act to expedite development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need. Pretomanid is only the third tuberculosis drug to receive FDA approval in more than 40 years.[3][8]
PATENT
IN 201641030408
HETERO RESEARCH FOUNDATION
http://ipindiaservices.gov.in/PatentSearch/PatentSearch/ViewPDF
- By Reddy, Bandi Parthasaradhi; Reddy, Kura Rathnakar; Reddy, Adulla Venkat Narsimha; Krishna, Bandi Vamsi
- From Indian Pat. Appl. (2018), IN 201641030408
The nitroimidazooxazine Formula I (PA-824) is a new class of bioreductive drug for tuberculosis. The recent introduction of the nitroimidazooxazine Formula I (PA-824) to clinical trial by the Global Alliance for TB Drug Development is thus of potential significance, since this compound shows good in vitro and in vivo activity against Mycobacterium tuberculosis in both its active and persistent forms. Tuberculosis (TBa) remains a leading infectious cause of death worldwide, but very few new drugs have been approved for TB treatment ifi the past 35 years, the current drug therapy for TB is long and complex, involving multidrug combinations.
The mechanism of actiém of Pretomanid is thoughrto involve reduction of the nitro group, in a‘ process dependent on the Bacterial ‘ m E Nfilw‘fieéFPEOEPEa‘e fillyeifiaasnfi (F8189); $943“; 20mm; “q Mcyarecent Swiss on mutant strains showed that a 151-amino acid (17.37 kDa) protein of unknown function, Rv3547, also, appears to be critical for this activation. Equivalent genes are present in M. boVis and MaVium.
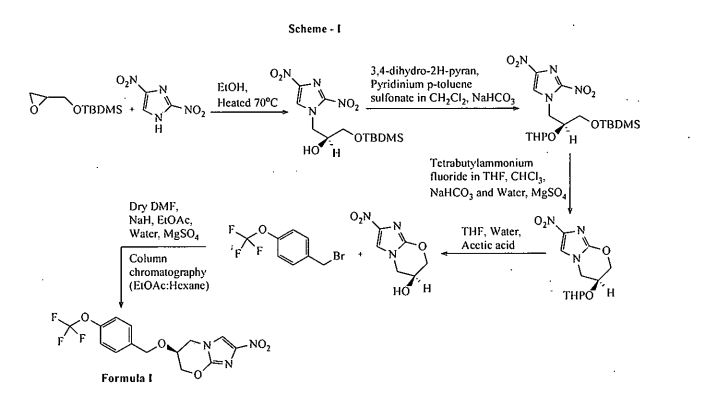
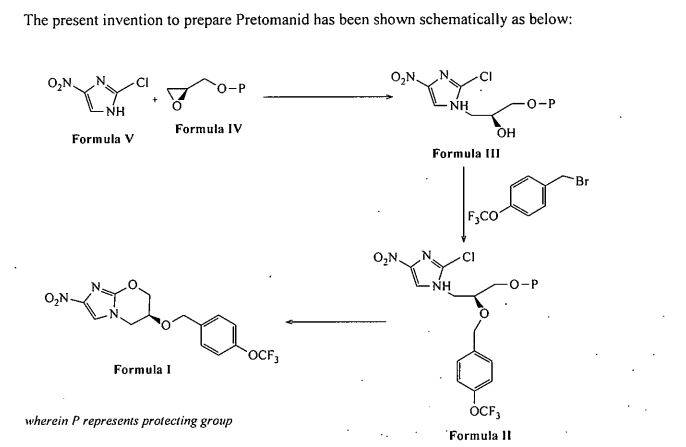
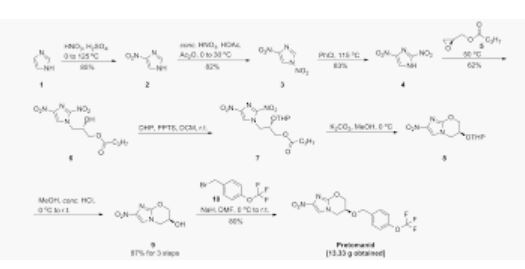
Pretomanid and its pharmace’utically acceptable salts Were generically disclosed in US 5,668,127 A and Specifically disclosed in US 6,087,358 A. US ‘358 patent discloses a process for the preparation of Pretomanid, which is as shown below in scheme 1:
CN 104177372 A discloses a process for the preparation of Pretomanid, which is as shown below in scheme II: 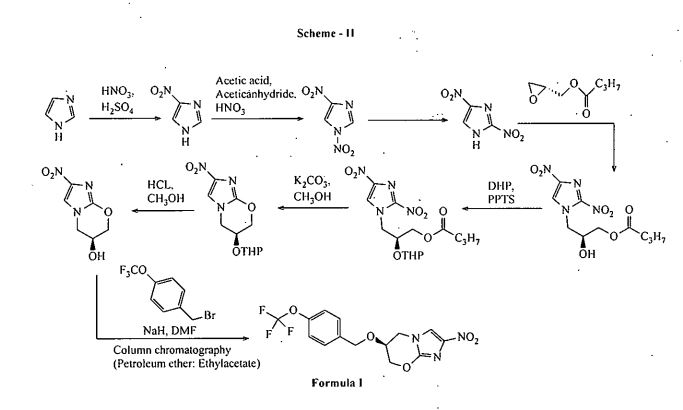
Bioorganic & Medicinal Chemistry Letters 2008, Volume: 18, Issue: 7, Pages: 2256-2262 discloses a process for the preparation of Pretomanid, which is as shown below in scheme Ill: 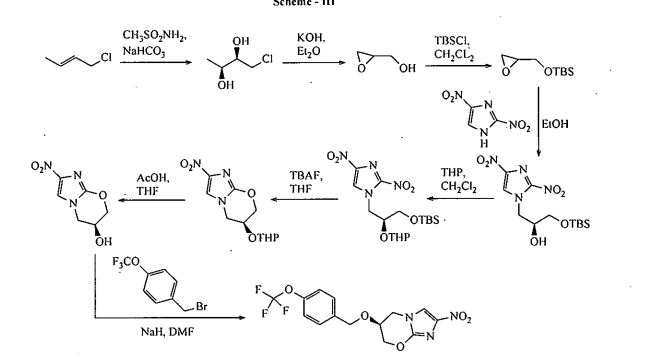
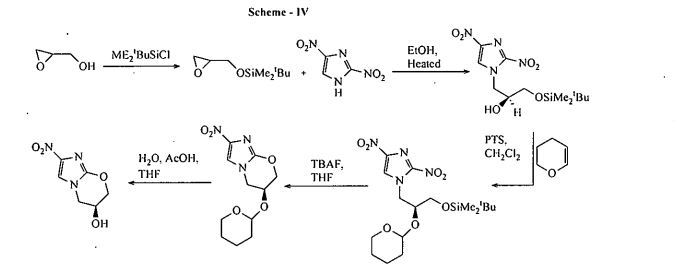
US 7,!15,736 B2-discloses_a process fdr the preparation of 3S-Hydroxy-6-nitrQ-2H-3, 4— dihydro-[2-1b]-imidazopyran which is a key intermediate of Pretomanid, which is as shown below in scheme IV:
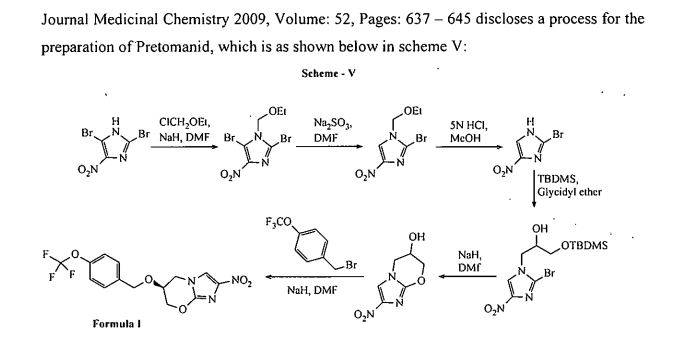
Journal Medicinal Chemistry 2009, Volume: 52, Pages: 637 — 645 discloses a process for the preparation of ‘Pretomanid, which is as shown below in scheme V:
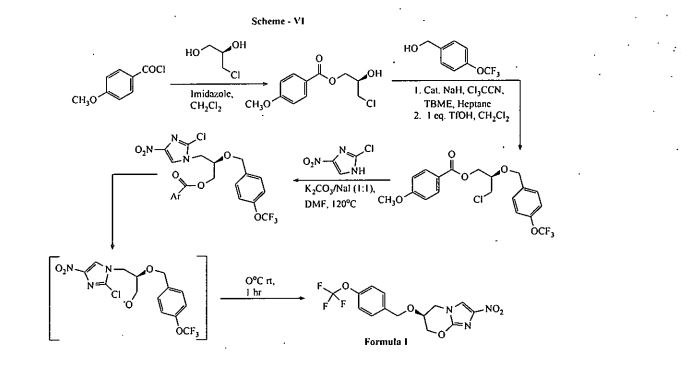
Joumal Organic Chemistry 2010; Volume: 75 (2]), Pages: 7479—82 discloses a process for. the preparation of Pretomanid, which is as shown below in scheme VI:
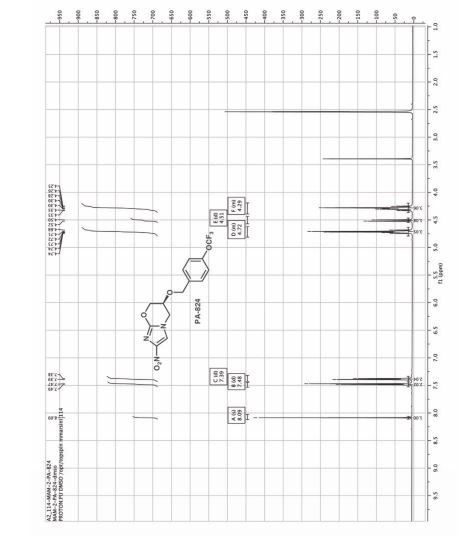
Example 3: Preparation of Pretomanid (S) 1- -(3 (tert- -Butyldomethylsilyloxy)- -2- -(-4 -(trifluoromethoxy)-71benzyloxy2‘- propyl)- 2- -methylP AT E N4Tnitro- fi-Eimigazole (Efgm Awlas (3315;501:1691 gin! %etra%1y7drofuraen (18(150 ml) at room temperature and stirred for 5 to 10 minutes then TBAF (9516 ml) was added to the reaction mixture and stirred for 2 hours, at room temperature, afler completion of the reaction removed solvent through vacuum to obtained residue, dissolved the residue in MDC (1800 ml) and water (1800 ml) stirred, separated the layers and the organic layer washed with 10% ‘ sodium bicarbonate the obtained organic solution was concentrated under atmospheric pressure to obtained residue added MeOH (1730 ml) at room temperature and the reaction mixture was cooled to 0°C to 5°C, added KOH (24.5 gm) at the same temperaturé then cooled to room temperature and stirred for 24 hours. After completion of reaction DM Water added drop wise over 30 minutes at 10°C to 15° C and stirred for 1 hour to 1 hour 30 minutes at room’lemperature, filtrated the compound and washed with DM wa‘er (133 ml) and dried under vacuum for 10 hours at 50° C. Yield: 53 gm , Chromatographic purity: 97.69% (by HPLC):
Example 4: Purification of Pretomanid Pretomanid (53 gm) was dissolved in MDC (795 ml) at room temperatur’e and stirred for 10 to 15 minutes, added charcoal (10 gm) and stirred for 30-35 minutes, remove the charcoal and concentrated to obtained residue: Dissolved the obtained residue in IPA (795 ml) and the reaction mixture was heated to 80°C maintained for 10-15 minutes, added cyclohexane (1600ml) for 30 minutes at 80° C, then cooled to room temperature and stirred the reaction mass for overnight, filtered the solid and washed with cyclohexane (265 ml), and dried under vacuum for 10 hours at 50° C. Yield: 48 gm (Percentage of Yield: 90%) Chromatographic purity: 99.97% by HPLC).
CLIP

CN104177372A.
WO9701562A1.
IN 201641030408
IN 201621026053
CN 107915747
CN 106632393
CN 106565744
CN 104177372
WO 9701562
US 6087358
PAPER
Science (Washington, DC, United States) (2008), 322(5906), 1392-1395.
Paper
PAPER
Huagong Shikan (2010), 24(4), 32-34, 51.
Xiaojin; Bioorganic & Medicinal Chemistry Letters 2008, Vol 18(7), Pg 2256-2262
PAPER
Orita, Akihiro; Advanced Synthesis & Catalysis 2007, Vol 349(13), Pg 2136-2144
https://onlinelibrary.wiley.com/doi/abs/10.1002/adsc.200700119
https://application.wiley-vch.de/contents/jc_2258/2007/f700119_s.pdf
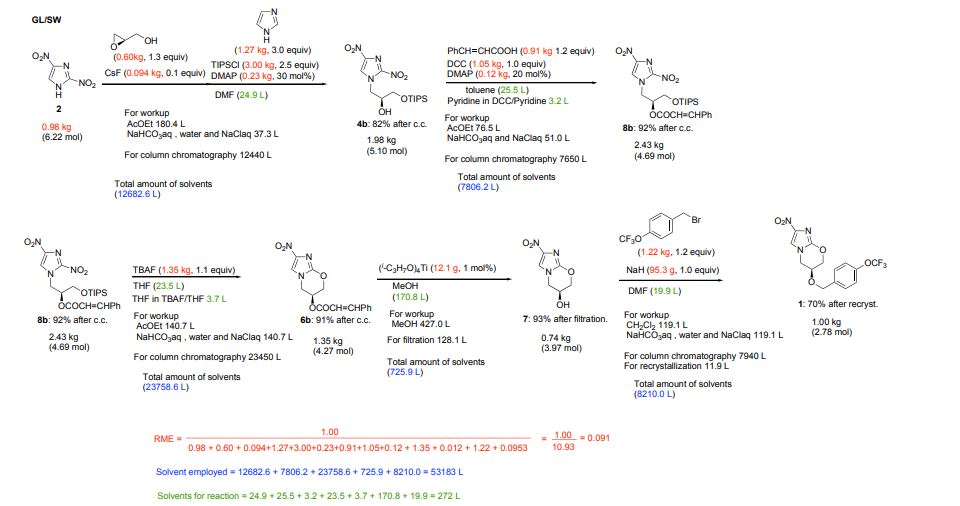
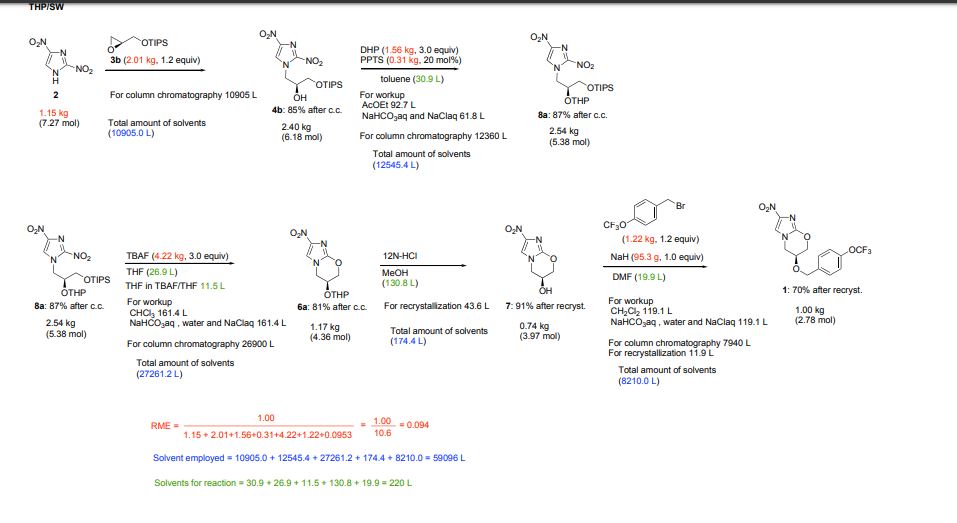
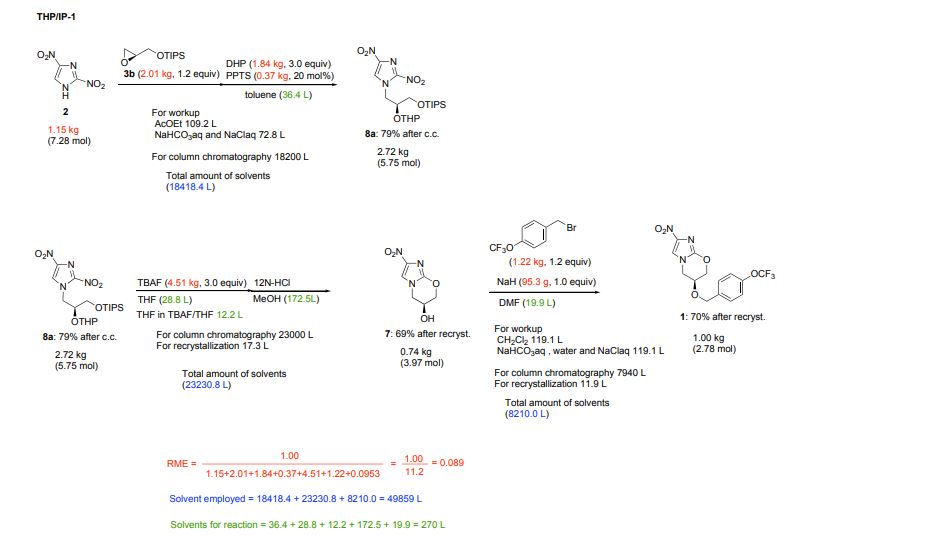
Marsini, Maurice A.; Journal of Organic Chemistry 2010, Vol 75(21), Pg 7479-7482

Scheme 1

aDHP = 3,4-dihydropyran; p-TsOH = p-toluenesulfonic acid; MsOH = methanesulfonic acid.
Scheme 3

aCl3CCN = trichloroacetonitrile; TBME = tert-butylmethyl ether; TfOH = trifluoromethanesulfonic acid.

PAPER
Journal of Medicinal Chemistry (2010), 53(1), 282-294.
Journal of Medicinal Chemistry (2009), 52(3), 637-645.
PATENT
References
- ^ Jump up to:a b c d e “FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs”. FDA. 14 August 2019. Retrieved 28 August 2019.
- ^ “Compounds | TB Alliance”. www.tballiance.org. Retrieved 2019-04-18.
- ^ Jump up to:a b Abutaleb Y (14 August 2019). “New antibiotic approved for drug-resistant tuberculosis”. Washington Post.
- ^ “TB Medicine Pretomanid Enters Regulatory Review Process in the United States | TB Alliance”. www.tballiance.org. Retrieved 2019-04-18.
- ^ “About TB Alliance”. TB Alliance. Retrieved 2019-04-18.
- ^ “PA-824 has a New Generic Name: Pretomanid”. TB Alliance. Retrieved 2019-04-18.
- ^ Jump up to:a b Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, Rose JD, Reynolds RC, Orme IM (June 2005). “Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models”. Antimicrobial Agents and Chemotherapy. 49 (6): 2294–301. doi:10.1128/AAC.49.6.2294-2301.2005. PMC 1140539. PMID 15917524.
- ^ FDA News Release. FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs.
 |
|
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C14H12F3N3O5 |
| Molar mass | 359.261 g·mol−1 |
| 3D model (JSmol) | |
//////////////Pretomanid, FDA 2109, プレトマニド , Antibacterial, tuberculostatic, PA-824, ANTI tuberculostatic














