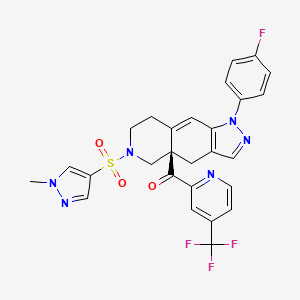
Relacorilant
- Molecular FormulaC27H22F4N6O3S
- Average mass586.561 Da
CAS 1496510-51-0
Phase III
[(4aR)-1-(4-fluorophenyl)-6-(1-methylpyrazol-4-yl)sulfonyl-4,5,7,8-tetrahydropyrazolo[3,4-g]isoquinolin-4a-yl]-[4-(trifluoromethyl)pyridin-2-yl]methanone
- OriginatorCorcept Therapeutics
- ClassAntineoplastics; Fluorine compounds; Isoquinolines; Ketones; Organic sulfur compounds; Pyrazoles; Pyridines; Small molecules
- Mechanism of ActionGlucocorticoid receptor antagonists
- Orphan Drug StatusYes – Pancreatic cancer; Cushing syndrome
- Phase IIICushing syndrome; Ovarian cancer; Pancreatic cancer
- Phase IIFallopian tube cancer; Peritoneal cancer; Prostate cancer
- Phase I/IISolid tumours
- Phase IAdrenocortical carcinoma
Most Recent Events
- 09 Sep 2022Subgroup analysis efficacy data from a phase-II trial in Ovarian cancer presented at the 47th European Society for Medical Oncology Congress (ESMO-2022)
- 29 Jun 2022Phase-III clinical trials in Ovarian cancer (Combination therapy, Recurrent, Second-line therapy or greater) in USA (PO)
- 06 Jun 2022Corcept Therapeutics announces intentions to submit a NDA for Ovarian cancer
Relacorilant (developmental code name CORT-125134) is an antiglucocorticoid which is under development by Corcept Therapeutics for the treatment of Cushing’s syndrome.[1] It is also under development for the treatment of solid tumors and alcoholism.[1][2] The drug is a nonsteroidal compound and acts as an antagonist of the glucocorticoid receptor.[1] As of December 2017, it is in phase II clinical trials for Cushing’s syndrome and phase I/II clinical studies for solid tumors, while the clinical phase for alcoholism is unknown.[1]
Relacorilant is an orally available antagonist of the glucocorticoid receptor (GR), with potential antineoplastic activity. Upon administration, relacorilant competitively binds to and blocks GRs. This inhibits the activity of GRs, and prevents both the translocation of the ligand-GR complexes to the nucleus and gene expression of GR-associated genes. This decreases the negative effects that result from excess levels of endogenous glucocorticoids, like those seen when tumors overproduce glucocorticoids. In addition, by binding to GRs and preventing their activity, inhibition with CORT125134 also inhibits the proliferation of GR-overexpressing cancer cells. GRs are overexpressed in certain tumor cell types and promote tumor cell proliferation.
CLIP
https://europepmc.org/article/pmc/pmc8175224
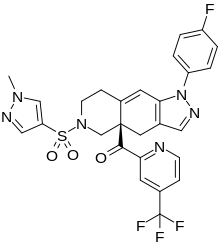
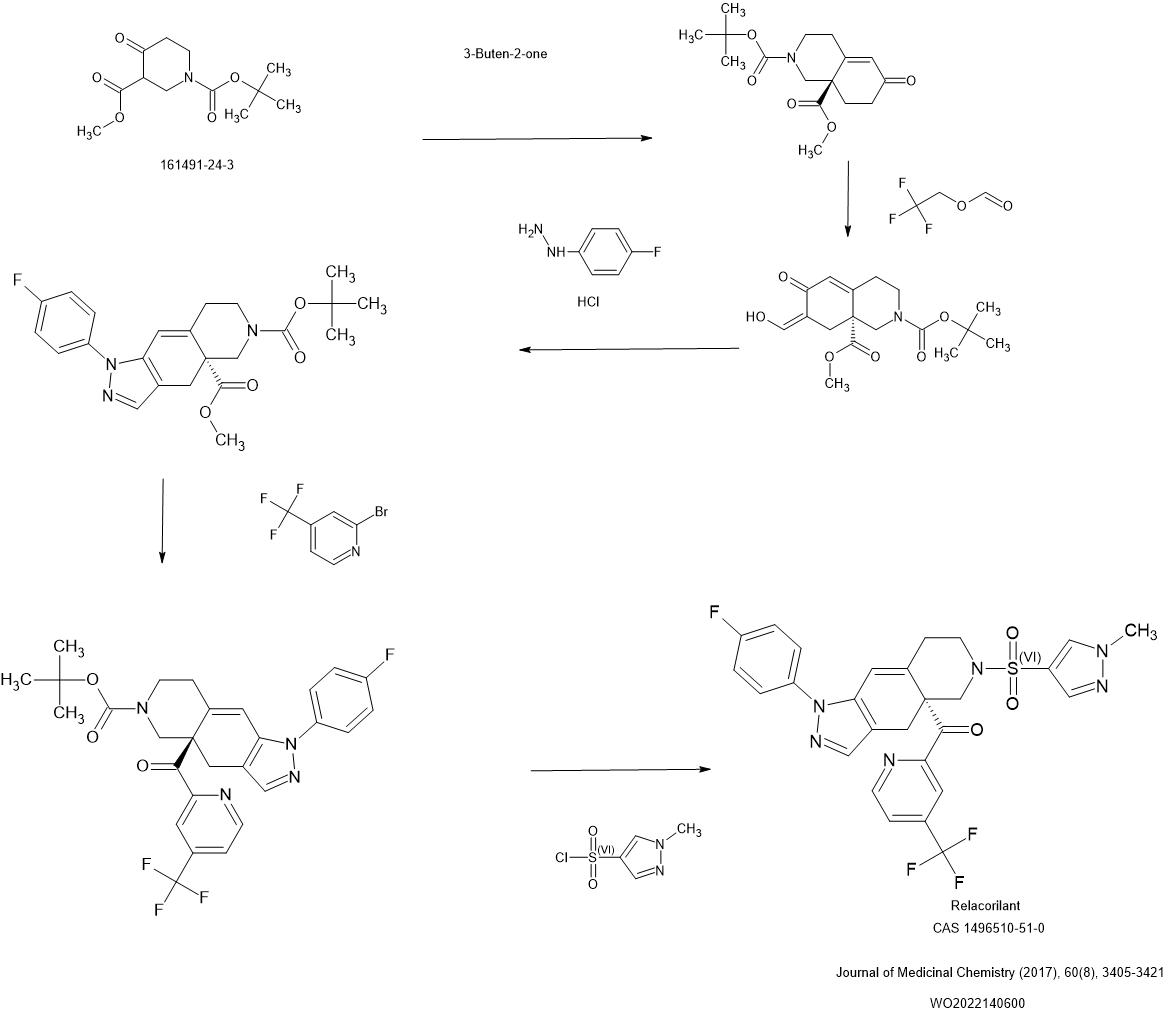
Relacorilant (CORT125134)118) is being developed by Corcept Therapeutics, Inc. It is an orally active, high-affinity, selective antagonist of the glucocorticoid receptor that may benefit from the modulation of cortisol activity. In structural optimization, the introduction of a trifluoromethyl group to the 4-position on the pyridyl moiety was found to increase HepG2 tyrosine amino transferase assay potency by a factor of four. Relacorilant is currently being evaluated in a phase II clinical study in patients with Cushing’s syndrome.119)
2-Bromo-4-(trifluoromethyl)pyridine (17) prepared from (E)-4-ethoxy-1,1,1-trifluorobut-3-en-2-one is employed as a key intermediate for the preparation of relacorilant as shown in Scheme 31.120)

 31. Synthesis of relacorilant.118)
31. Synthesis of relacorilant.118)
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013177559
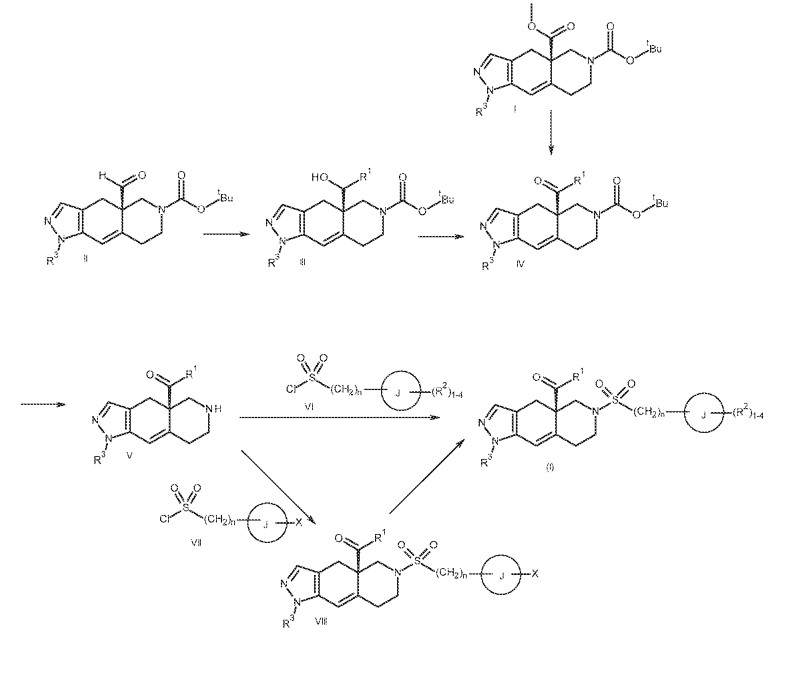
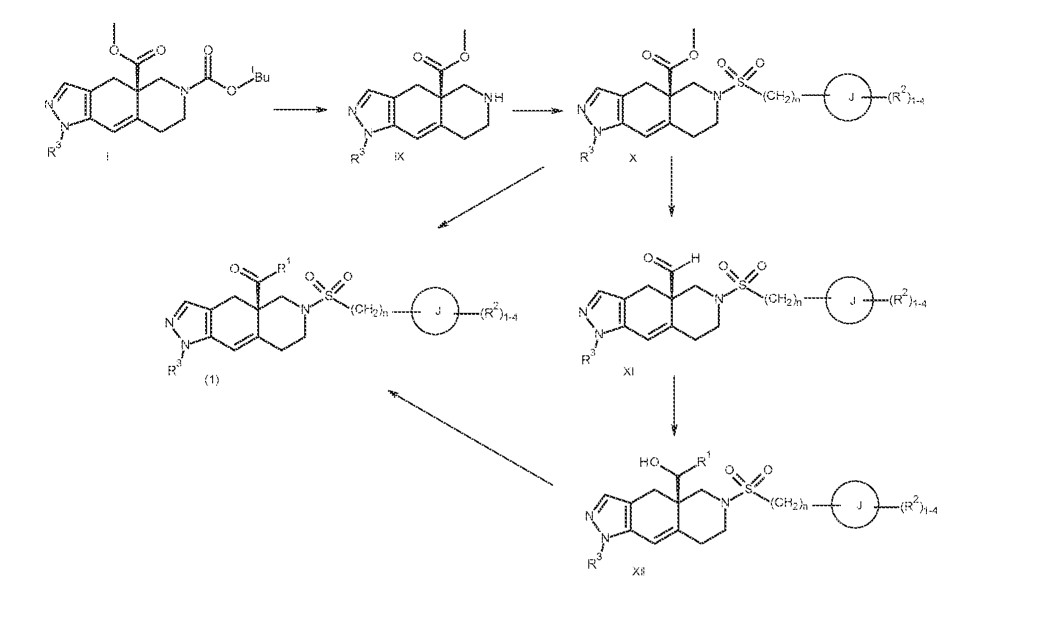
SYN
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Cushing’s syndrome (CS) is a metabolic disorder caused by chronic hypercortisolism. CS is associated with cardiovascular, metabolic, skeletal and psychological dysfunctions and can be fatal if left untreated. The first-line treatment for all forms of CS is a surgery. However, medical therapy has to be chosen if surgical resection is not an option or is deemed ineffective. Currently available therapeutics are either not selective and have side effects or are only available as an injection (pasireotide).
References
- ^ Jump up to:a b c d “Relacorilant – Corcept Therapeutics – AdisInsight”.
- ^ Veneris JT, Darcy KM, Mhawech-Fauceglia P, Tian C, Lengyel E, Lastra RR, Pejovic T, Conzen SD, Fleming GF (2017). “High glucocorticoid receptor expression predicts short progression-free survival in ovarian cancer”. Gynecol. Oncol. 146 (1): 153–160. doi:10.1016/j.ygyno.2017.04.012. PMC 5955699. PMID 28456378.
External links
 |
|
| Clinical data | |
|---|---|
| Other names | CORT-125134 |
| Routes of administration |
By mouth |
| Drug class | Antiglucocorticoid |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C27H22F4N6O3S |
| Molar mass | 586.57 g·mol−1 |
| 3D model (JSmol) | |
//////////////Relacorilant, Phase III , Orphan Drug, Cushing syndrome, Ovarian cancer, Pancreatic cancer, релакорилант , ريلاكوريلانت , 瑞拉可兰 ,
CN1C=C(C=N1)S(=O)(=O)N2CCC3=CC4=C(CC3(C2)C(=O)C5=NC=CC(=C5)C(F)(F)F)C=NN4C6=CC=C(C=C6)F