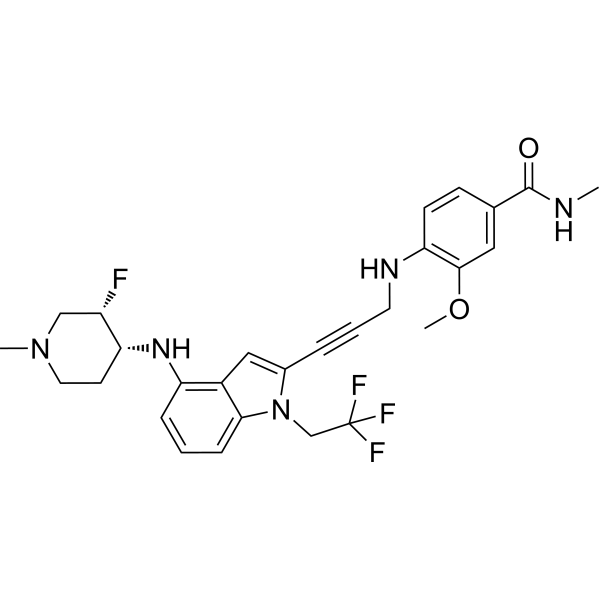
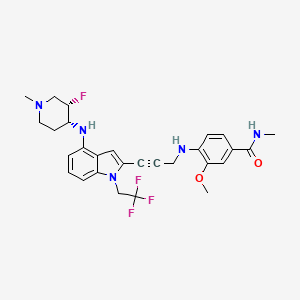
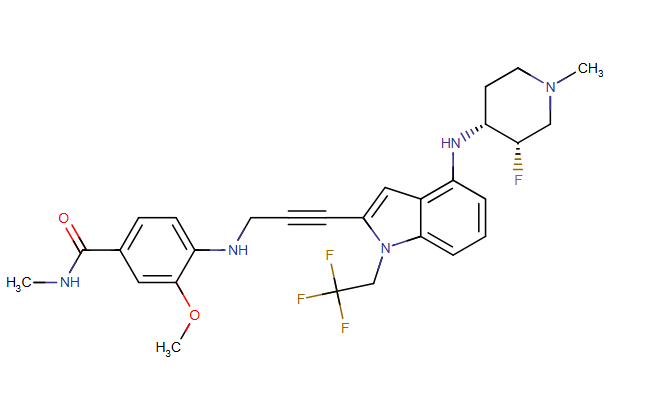
Rezatapopt, PC 14586
CAS 2636846-41-6
| Molecular Weight | 545.57 |
|---|---|
| Synonyms | PC14586 |
| Formula | C28H31F4N5O2 |
| CAS No. | 2636846-41-6 |
4-[3-[4-[[(3S,4R)-3-fluoro-1-methylpiperidin-4-yl]amino]-1-(2,2,2-trifluoroethyl)indol-2-yl]prop-2-ynylamino]-3-methoxy-N-methylbenzamide
- 4-[3-[4-[[(3S,4R)-3-fluoro-1-methylpiperidin-4-yl]amino]-1-(2,2,2-trifluoroethyl)indol-2-yl]prop-2-ynylamino]-3-methoxy-N-methylbenzamide
- Benzamide, 4-[[3-[4-[[(3S,4R)-3-fluoro-1-methyl-4-piperidinyl]amino]-1-(2,2,2-trifluoroethyl)-1H-indol-2-yl]-2-propyn-1-yl]amino]-3-methoxy-N-methyl-
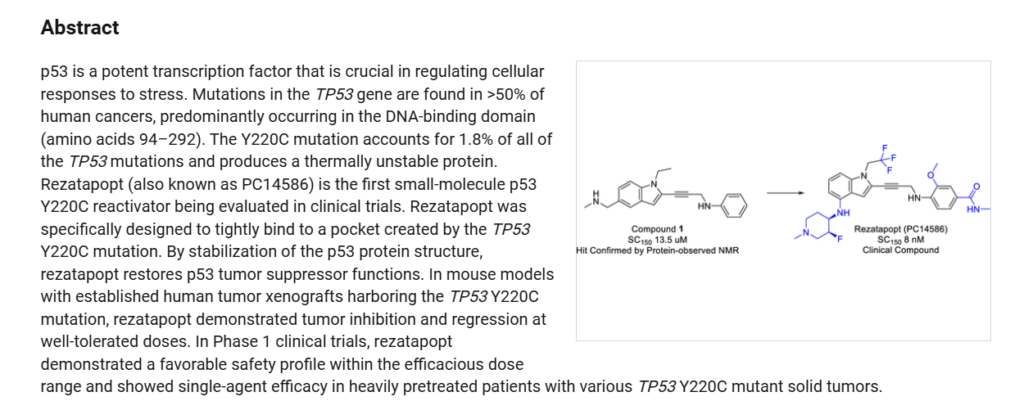
Rezatapopt (PC14586) is an orally active antineoplastic agent. Rezatapopt binds to a pocket created by the TP53 Y220C mutation. Rezatapopt restores p53 tumor suppressor functions by stabilization of the p53 protein structure. Rezatapopt demonstrates tumor inhibition and regression in mouse models with established human tumor xenografts harboring the TP53 Y220C mutation.
SCHEME
COUPLER
COUPLER
MAIN
REF
PAPER
https://pubs.acs.org/doi/10.1021/acsmedchemlett.4c00379
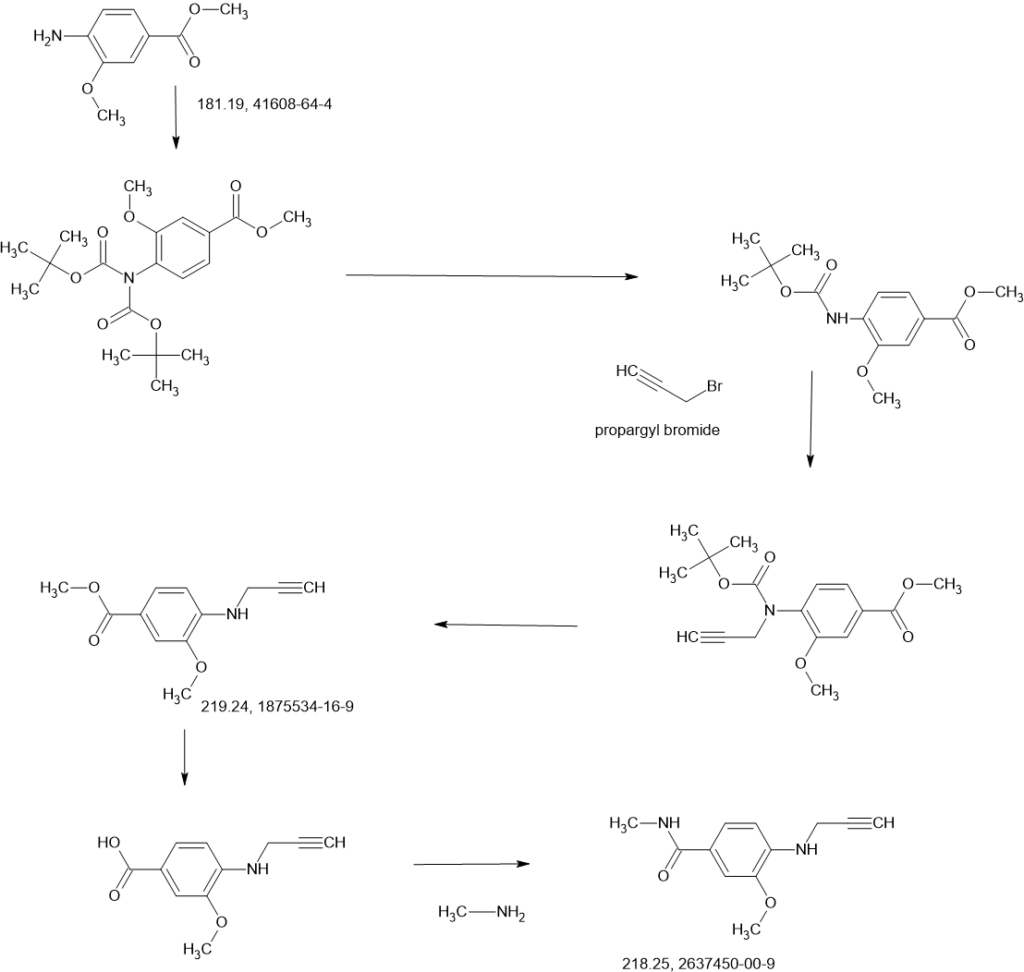
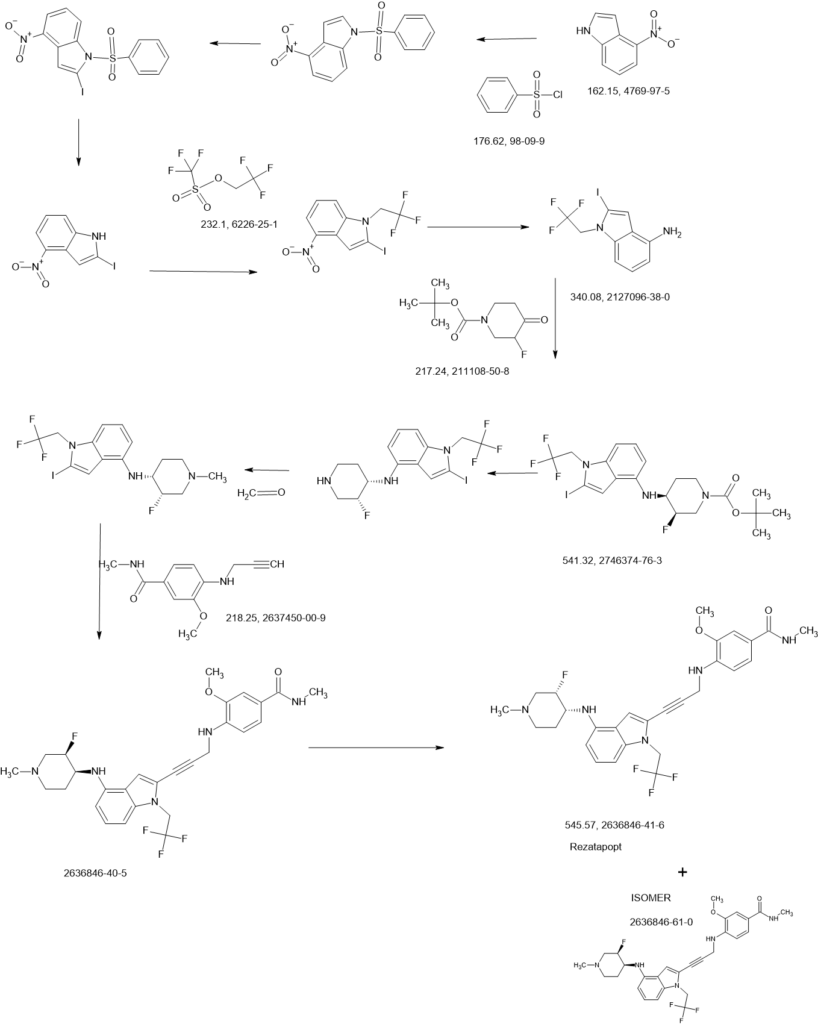
2-Iodo-1-(2,2,2-trifluoroethyl)-1H-indol-4-amine 15 was prepared from 4-nitroindole as described in
WO2017143291. 1
H NMR (400 MHz, dimethylsulfoxide [DMSO]-d6) δ ppm 9.19–10.88 (m, 2 H), 7.63
(d, J=8.34 Hz, 1 H), 7.16–7.25 (m, 1 H), 7.04–7.14 (m, 2 H), 5.14–5.33 (m, 2 H). LCMS (ES+
, m/z):
340.9 [(M+H)+
].
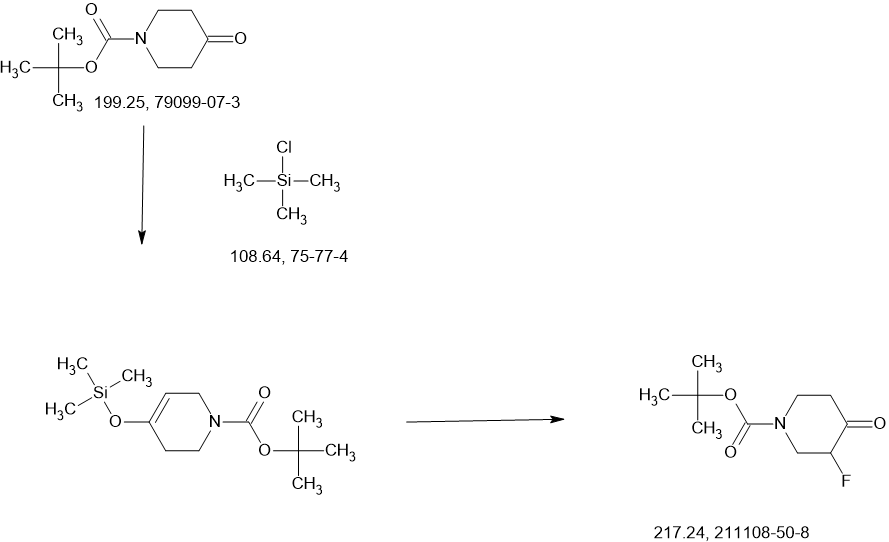
SnCl2.2H2O (398.11 mg, 1.76 mmol, 0.20 eq.) was added to a solution of 2-iodo-1-(2,2,2-trifluoroethyl)-
1H-indol-4-amine 15 (3.00 g, 8.82 mmol, 1.00 eq.) and 1-methylpiperidin-4-one (1.20 g, 10.61 mmol,
1.20 eq.) in MeOH (10.00 mL). The mixture was stirred at 25 °C for 3 hours (h), and then NaBH3CN
(2.77 g, 44.1 mmol, 5.00 eq.) was added, stirring at 25 °C for 69 h. Thin-layer chromatography (TLC)
indicated that the starting material was consumed, and the reaction mixture was filtered. The filtrate was
poured into H2O (200 mL) and extracted with ethyl acetate ([EtOAc] 200 mL2). The combined organic layers were washed with H2O (200 mL), dried over Na2SO4, and concentrated under reduced pressure to give a residue. The crude material was purified by flash column chromatography (Silica gel, petroleum ether (PE) : EtOAc = 0:1) and then by preparative high performance chromatography ([prep-HPLC] column: Phenomenex Luna C18 10040mm5 um; mobile phase: [H2O (0.2% Formic acid-acetonitrile [ACN])]; gradient: 10%–50% acetonitrile over 8.0 minutes) to yield 2-iodo-N-(1-methylpiperidin-4-yl)-1- (2,2,2-trifluoroethyl)-1H-indol-4-amine 16 (2.50 g, 5.72 mmol, 64.93% yield) as a light-brown solid. 1 H NMR (400 MHz, DMSO-d6) δ ppm 8.24 (br s, 1 H, formic acid salt), 7.17 (s, 1 H), 6.85–6.95 (m, 1 H), 6.78 (br d, J = 7.99 Hz, 1 H), 6.16 (br d, J = 7.63 Hz, 1 H), 5.44 (br d, J = 2.62 Hz, 1 H), 4.99 (q, J = 8.54 Hz, 2 H), 3.33 (br s, 1 H), 2.85 (br d, J = 9.66 Hz, 2 H), 2.25 (br s, 3 H), 2.07–2.18 (m, 2 H), 1.94 (br d, J = 12.04 Hz, 2 H), 1.46–1.58 (m, 2 H). LCMS (ES+, m/z): 438.0 [(M+H)+]. Boc2O (26.03 g, 119.26 mmol, 6.00 eq.) was added to a solution of 2-methoxy-4-(methylsulfonyl)aniline 17 (4.00 g, 19.88 mmol, 1.00 eq.) in dioxane (40.00 mL) at 25 o C (room temperature). The reaction mixture was stirred at 110 °C for 16 h. TLC and LCMS indicated that the reaction was completed, and it was concentrated in vacuo. The residue was purified by column chromatography (SiO2, PE/EtOAc = 10/1 to 1:1) to yield tert-butyl (2-methoxy-4-(methylsulfonyl)phenyl)carbamate (6.00 g, 19.92 mmol, 72.6% purity, 72% yield) as a yellow gum. 1 H NMR (400 MHz, DMSO-d6) δ ppm 8.33 (s, 1 H), 8.03 (d, J = 8.38 Hz, 1 H), 7.47 (dd, J = 8.38, 2.00 Hz, 1 H), 7.44 (d, J = 2.00 Hz, 1 H), 3.91 (s, 3 H), 3.18 (s, 3 H), 1.47 (s, 9 H). LCMS (ES+, m/z): 324.1 [(M+Na)+ ]. NaH (867.27 mg, 60% purity, 21.69 mmol, 3.00 eq.) was added in portions at 0 °C to a mixture of tert-butyl (2-methoxy-4-(methylsulfonyl)phenyl)carbamate (3.00 g, 7.23 mmol, 1.00 eq.) in dimethylformamide ([DMF] 30.00 mL) and stirred at 0 °C for 0.5 h. 3-Bromoprop-1-yne (3.23 g, 21.69 mmol, 3.00 eq.) was added to the reaction mixture, stirring at 0 °C for 2.5 h. TLC (Plate 1: PE : EtOAc = 1:1) and LCMS indicated that the starting material was consumed, and the product was detected. The reaction mixture was poured into a saturated solution of NH4Cl (200 mL) at 0 o C and was extracted with EtOAc (200 mL3). The combined organic phase was dried over Na2SO4, filtered, and concentrated
in vacuo. The residue was purified by column chromatography (SiO2, PE : EtOAc = 5:1 to 1:2) to give
tert-butyl (2-methoxy-4-(methylsulfonyl)phenyl)(prop-2-yn-1-yl)carbamate (3.00 g, 8.85 mmol,
74% purity, 90% yield) as a light-yellow gum.
1
H NMR (400 MHz, DMSO-d6) δ ppm 7.53–7.56 (m, 1 H), 7.46–7.53 (m, 2 H), 4.10–4.51 (m, 2 H), 3.90
(s, 3 H), 3.27 (s, 3 H), 3.17 (t, J = 2.32 Hz, 1 H), 1.27–1.39 (m, 9 H). LCMS (ES+
, m/z): 283.9 [(M+H-tBu)+].
A solution of 4M HCl/EtOAc (20.00 mL) was added to the solution of tert-butyl (2-methoxy-4-
(methylsulfonyl)phenyl)(prop-2-yn-1-yl)carbamate (3.00 g, 6.54 mmol, 1.00 eq.) in EtOAc (1.00 mL).
The reaction mixture was stirred at 25 °C for 2 h. TLC indicated that the starting material was consumed
completely. The reaction mixture was concentrated in vacuo to yield 2-methoxy-4-(methylsulfonyl)-N-
(prop-2-yn-1-yl)aniline 18 (1.80 g, 7.53 mmol, 85.3% yield, HCl salt) as a yellow solid.
1
H NMR (400 MHz, DMSO-d6) δ ppm 7.38 (dd, J = 8.40, 1.60 Hz, 1 H), 7.22 (d, J = 1.60 Hz, 1 H), 6.75
(d, J = 8.80 Hz, 1 H), 3.99 (d, J = 2.4 Hz, 2 H), 3.87 (s, 3 H) 3.10 (s, 3 H), 3.08 (t, J = 2.31 Hz, 1 H).
LCMS (ES+
, m/z): 240.1 [(M+H)+
].
i-Pr2NH (2.08 g, 20.58 mmol, 2.91 mL, 10 eq.), CuI (392.02 mg, 2.06 mmol, 1 eq), 2-iodo-N-(1-
methylpiperidin-4-yl)-1-(2,2,2-trifluoroethyl)-1H-indol-4-amine 16 (0.9 g, 2.06 mmol, 1 eq.) and
Pd(PPh3)4 (475.71 mg, 411.67 μmol, 0.2 eq.) was added to a solution of 2-methoxy-4-(methylsulfonyl)-N-
(prop-2-yn-1-yl)aniline 18 (622.16 mg, 2.47 mmol, 1.2 eq.) in DMSO (10 mL) at 45 °C under N2. The
reaction mixture was stirred at 45 °C for 1 h. TLC (DCM/MeOH=10:1, Rf = 0.3) indicated that the
starting material was consumed completely. It was poured into ethylenediaminetetraacetic acid ([EDTA]
20 mL) and stirred for 1 h, then extracted with EtOAc (40 mL3). The combined organic phase was washed with brine (40 mL), dried with anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (SiO2, PE : EtOAc = 1:1 to dichloromethane (DCM) / MeOH = 10:1, Rf = 0.3), then by prep-HPLC (column: Phenomenex Luna(2) C18 25050 10u; mobile
phase: [water (0.1% trifluoroacetic acid)-ACN]; B%: 30%–50%, 20 min) to yield compound 13 (0.6 g,
1.09 mmol, 53.08% yield, 99.9% purity) as a light-yellow solid.
1 H NMR (400 MHz, DMSO-d6) δ ppm 1.41–1.54 (m, 2 H), 1.91 (br d, J = 11.00 Hz, 2 H), 1.95–2.08 (m,
2 H) 2.17 (s, 3 H), 2.68–2.80 (m, 2 H), 3.10 (s, 3 H), 3.20–3.29 (m, 1 H), 3.89 (s, 3 H), 4.36 (d,
J = 6.24 Hz, 2 H), 4.92 (q, J = 9.09 Hz, 2 H), 5.49 (d, J = 7.95 Hz, 1 H), 6.15 (d, J = 7.83 Hz, 1 H),
6.50 (t, J = 6.24 Hz, 1 H), 6.68 (d, J = 8.19 Hz, 1 H), 6.89 (d, J = 8.44 Hz, 1 H), 6.99 (t, J = 8.01 Hz,
1 H), 7.09 (s, 1 H), 7.25 (d, J = 1.83 Hz, 1 H), 7.39 (dd, J = 8.31, 1.83 Hz, 1 H). LCMS (ES+, m/z):
549.3 [(M+H)+
]
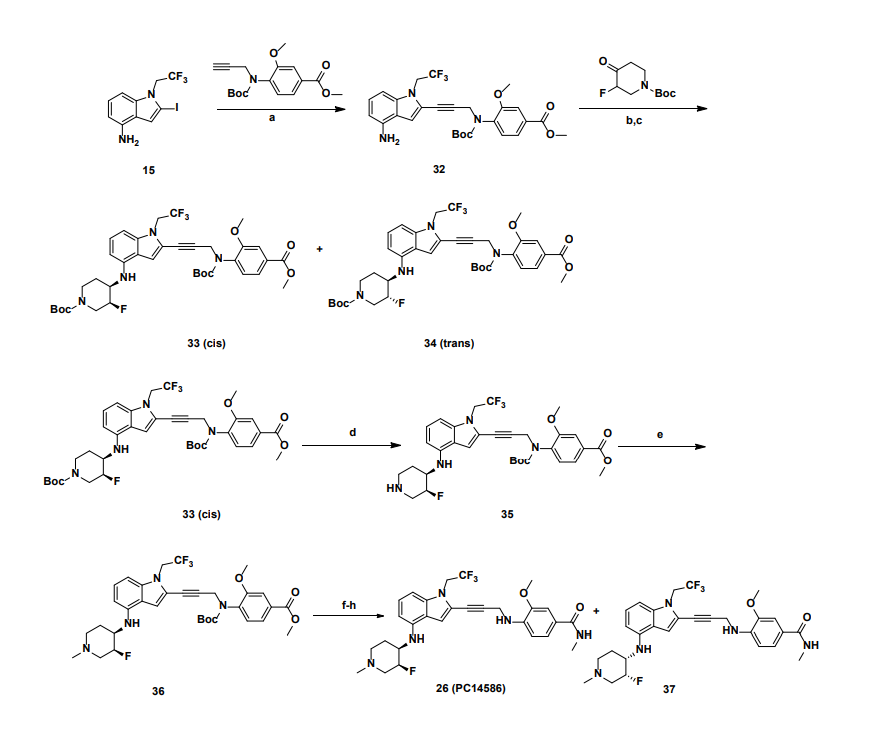
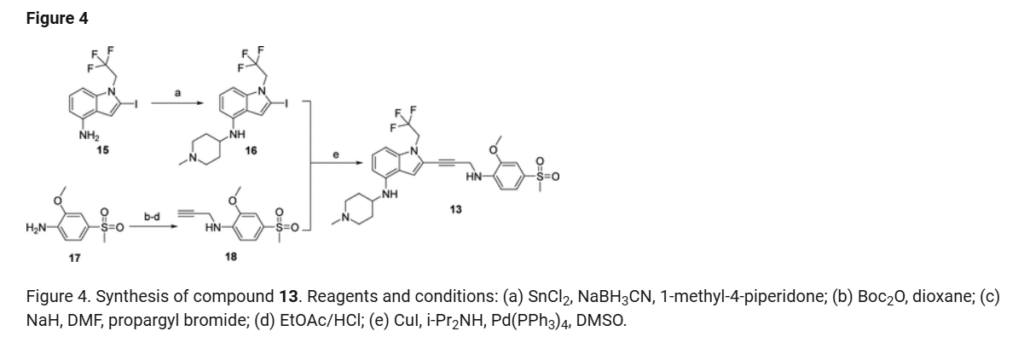
a
Reagents and conditions: (a) Pd(PPh3)4, CuI, diisopropylamine, DMSO, 20 °C, 1 h; (b) TMSCl, DMF, 0 °C, 0.5 h;
(c) BH3.THF, 0 °C, 0.5 h; (d) EtOAc/HCl, 20 °C, 1 h; (e) 10 eq. (CH2O)n, NaBH3CN, MeOH, 20 °C, 16 h; f)
LiOH.H2O, MeOH, 40 °C, 12 h; g) MeNH3Cl, HOBT, EDCI, TEA, DCM, RT, 16 h; h) Chiral SFC separation
PATENTS
WO2023016434 36%
WO2021061643
US20230024905
WO2023016434 Jacobio Pharmaceuticals Co., Ltd.
WO2023225477 PMV Pharmaceuticals, Inc.
US20230024905 PMV Pharmaceuticals, Inc.
WO2021061643 PMV Pharmaceuticals, Inc.
WO2021262483, PMV Pharmaceuticals, Inc.
WO2023196993 PMV Pharmaceuticals, Inc.
WO2021262484 WO2021262541
- [1]. Li Sujing, et al. Heteroarylalkyne compounds for targeting mutant of p53 and their preparation. World Intellectual Property Organization, WO2023016434 A1. 2023-02-16.[2]. Vu BT, et al. Discovery of Rezatapopt (PC14586), a First-in-Class, Small-Molecule Reactivator of p53 Y220C Mutant in Development. ACS Med Chem Lett. 2024 Nov 4;16(1):34-39. [Content Brief][3]. Spiegelberg D, et al. Targeting mutant p53: Evaluation of novel anti-p53R175H monoclonal antibodies as diagnostic tools. Sci Rep. 2025 Jan 6;15(1):1000. [Content Brief][4]. Schram A M, et al. 691TiP PYNNACLE phase II trial of rezatapopt (PC14586) in solid tumors with a TP53 Y220C mutation[J]. Annals of oncology, 2024, 35: S535-S536.
//////////Rezatapopt, PC 14586, 5W59S33KC9



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com














