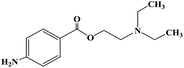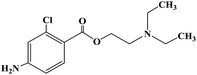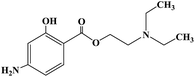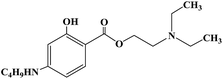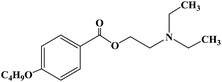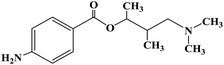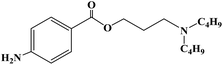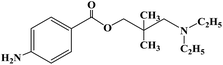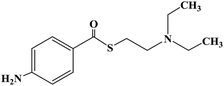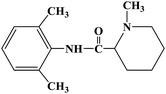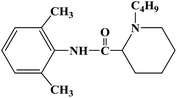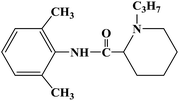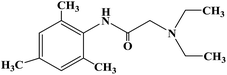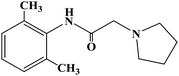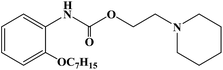
Ropivacaine
CAS No.84057-95-4 (Ropivacaine);
- Molecular FormulaC17H26N2O
- Average mass274.401 Da
HCL SALT
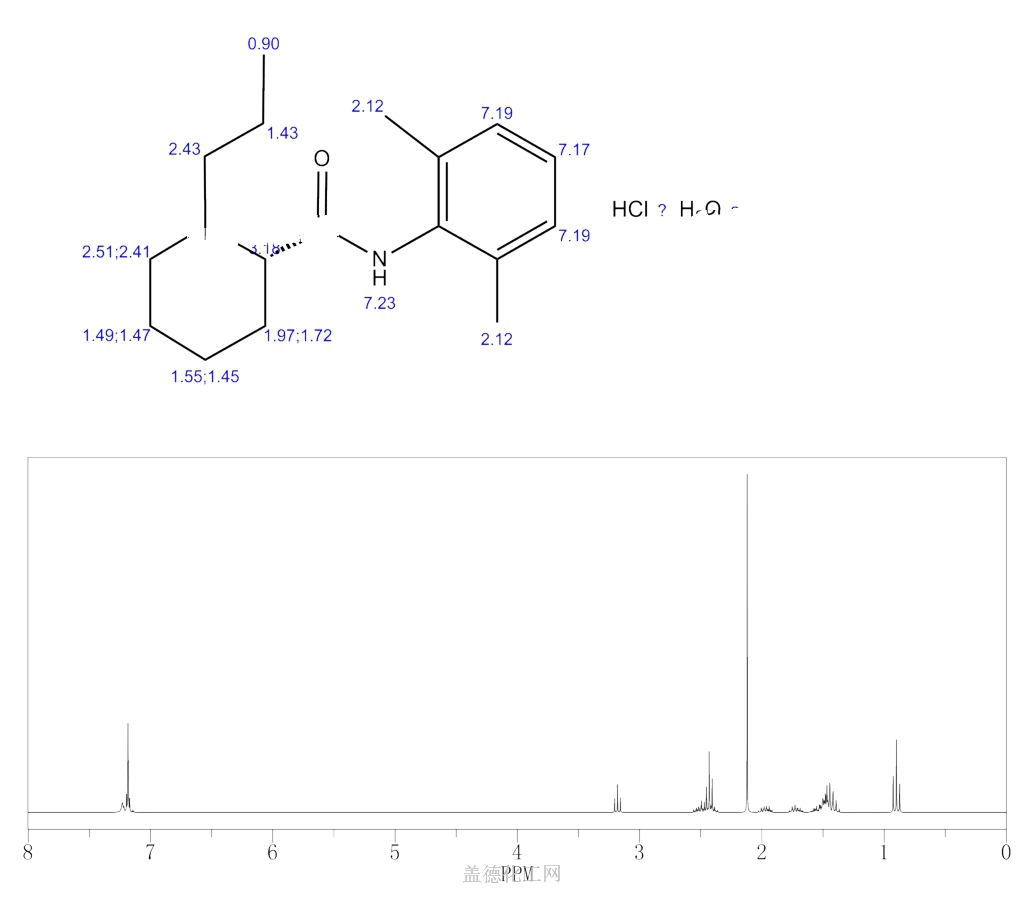
Molecular Weight328.88, FormulaC17H26N2O • HCl • H2O
132112-35-7 (Ropivacaine HCl Monohydrate);
Chemical Name S-(-)-1-propyl-2′,6′-pipecoloxylidide hydrochloride monohydrate
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 1996-09-26 | First approval | Naropin | Anaesthetic | Injection | 2 mg/ml; 5 mg/ml; 7.5 mg/ml; 10 mg/ml | APP Pharmaceuticals |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2001-04-04 | First approval | Anapeine | Anaesthetic | Injection | 2 mg/ml; 7.5 mg/ml; 10 mg/ml | AstraZeneca |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2010-02-11 | Marketing approval | 耐乐品/Naropin | Anaesthetic | Injection | 20 mg/10 ml;100 mg/10 ml; 75 mg/10 ml; 50 mg/10 ml | AstraZeneca | |
| 2010-02-03 | Marketing approval | 耐乐品/Naropin | Anaesthetic | Injection | 2 mg/mL | AstraZeneca |
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Ropivacaine hydrochloride | V910P86109 | 132112-35-7 | VSHFRHVKMYGBJL-CKUXDGONSA-N |
| Ropivacaine hydrochloride anhydrous | 35504LBE2T | 98717-15-8 | NDNSIBYYUOEUSV-RSAXXLAASA-N |
Ropivacaine hydrochloride hydrate was first approved by the U.S. Food and Drug Administration (FDA) on September 26, 1996, then approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) in April 4, 2001. It was developed by AstraZeneca, then marketed as Naropin® by APP Pharmaceuticals, LLC. in the US and as Anapeine® by AstraZeneca in JP.
Ropivacaine is a local anaesthetic drug belonging to the amino amide group. It is indicated for the production of local or regional anesthesia for surgery and for acute pain management.
Naropin® is available as injection solution for intravenous use, containing 2, 5, 7.5 or 10 mg of Ropivacaine hydrochloride one mL. Common concentration is 7.5 mg/mL, and the maximum single dose is 200 mg.
Ropivacaine (rINN) /roʊˈpɪvəkeɪn/ is a local anaesthetic drug belonging to the amino amide group. The name ropivacaine refers to both the racemate and the marketed S–enantiomer. Ropivacaine hydrochloride is commonly marketed by AstraZeneca under the brand name Naropin.

Syn
Synthesis Reference
Peter Jaksch, “Process for the preparation of ropivacaine hydrochloride monohydrate.” U.S. Patent US5959112, issued February, 1970.
1. WO8500599A1.
https://patents.google.com/patent/WO1985000599A1/en

1. CN104003930A.
https://patents.google.com/patent/CN104003930A/en
Literatures:
Navinta LLC Patent: US2006/276654 A1, 2006 ; Location in patent: Page/Page column 5 ;
Yield: ~82%
Literatures:
US2006/276654 A1, ; Page/Page column 5 ;
Yield: null
SYN
https://pubs.rsc.org/en/content/articlehtml/2019/ra/c9ra09287k
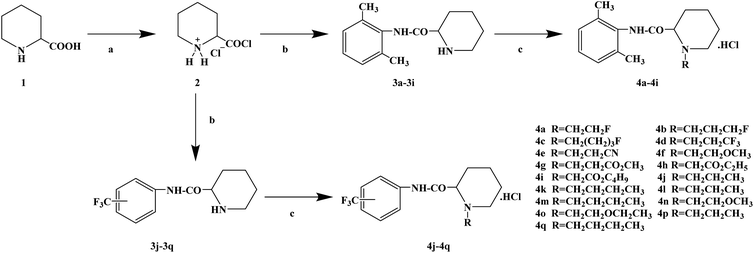
Ropivacaine is the S-enantiomer of an N-alkyl pipecoloxylidine derivative, which is the first local anesthetic with chiral activity, and is widely used in clinical infiltration anesthesia, conduction anesthesia and epidural anesthesia. It has a long of local anesthesia and analgesic effect. However, ropivacaine also has serious safety risks in clinical practice. When the concentration of ropivacaine in human blood is too high, it may cause toxicity to the cardiovascular and central nervous system, and even cause allergic reactions in some patients. Thus far, the mechanism of the effect of ropivacaine on local anesthesia is not clear. Ropivacaine is a multitarget drug that acts on the gamma-aminobutyric acid a receptor (GABAA-R) and N-methyl-D-aspartate acid receptor (NDMA-R). Sodium (Na+) channels are a key target of local anesthetics and these two receptors regulate sodium channels. Previous studies on the structural modification of ropivacaine mainly focused on the substitution of –CH3 on the phenyl group or the substitution of –CH2CH2CH3 on piperidine with different alkyl groups. In 2017, Wen L. et al.69 reported the design and synthesis of ropivacaine analogues for local anesthesia. In the process of structural design, they used ropivacaine as the lead compound to design two series of compounds, 4a–4q (17 new substituted imines). In the first series of compounds, 4a–4i, different substituents were selected to replace –CH2CH2CH3 on piperidine. In the second series of compounds, 4j–4q, the methyl groups were replaced by –CF3 at the o-positions, m-positions and p-positions. Meanwhile, the –CH2CH2CH3 on piperidine ring was also substituted and modified. The process for the synthesis of the target compounds is shown in Scheme 8. The synthetic route takes piperic acid (compound 1) as the starting material, hydrochloric acid and sulfoxide chloride as additives, and toluene as the reaction solvent to convert compound 1 into acyl chloride salt (compound 2). Compound 2 was then treated with substituted aniline and reacted at 58 °C for 5 h to form compounds 3a–3i and 3j–3q. Finally, bromoalkyl and hydrochloric acid were used to treat compounds 3a–3i and 3j–3q. Potassium carbonate (K2CO3) was used as an acid dressing agent and dimethylformamide (DMF) as the reaction solvent in N-alkylation reaction. The N-alkylation reaction lasted 10 h at 80 °C, and the salt reaction lasted 5 min at room temperature to obtain the final target compounds 4a–4q. The total yield of the target compounds ranged from 17.5% to 87.7%. The synthetic route has the advantages of mild reaction conditions, cheap reagents and simple operation. However, using this synthetic route, the total yield of some products is too low, and too low yield will bring great problems to the synthesis cost, which needs to be further optimized in follow-up work. In the evaluation of the local anesthesia effect, sciatic nerve block activity, infiltration anesthesia activity, corneal anesthesia activity and spinal cord anesthesia activity were used as evaluation indexes. Ropivacaine was used as a positive control substance to test the local anesthesia activity in vitro. Firstly, the local anesthesia effect of all the target compounds 4a–4q was screened by a sciatic nerve block test in toads in vitro (Table 6). The preliminary screening results in vitro showed that these compounds increased the blocking effect of the sciatic nerve on electrical stimulation, with ED50 values ranging from 0.012 to 0.64 (positive control for ropivacaine was 0.013), with the highest activity shown by compound 4b. In terms of latent period, that of target compounds 4a–4q ranged from 27.7 to 59.4 min. Based on the results of the preliminary in vitro screening, compounds 4a, 4b, 4c, 4j and 4l were selected to test the efficacy of invasive anesthesia in guinea pigs. The results of the infiltration anesthesia test showed that the local anesthetic effect of compounds 4c and 4l was similar to that of the positive control ropivacaine, and the local anesthetic activity of other compounds was lower than that of the positive control. Furthermore, compounds 4a, 4b, 4c, 4j and 4l were used to test the local surface anesthesia effect of these compounds (Table 7). The results of the surface anesthesia test showed that compound 4l had a similar local anesthetic effect as the positive control ropivacaine, while the effect of the other compounds was poor in comparison with the positive control. Finally, compounds 4a, 4b, 4c, 4j and 4l were tested for spinal anesthesia in order to further study their local surface anesthesia effect. The experimental results showed that the ED50 produced by compounds 4l and 4b was 5.02 and 7.87, respectively, while the effects of compounds 4a, 4c and 4j were poor. The evaluation of local anesthesia in vitro found that compound 4l had the best activity, and thus molecular docking of compound 4l and ropivacaine was conducted to further study its local anesthesia mechanism. The molecular docking results showed that compound 4l interacts with receptor proteins of VGSC, GABAA-R and NDMA-R, which may help optimize and predict the activity of these ropivacaine analogues as potential local anesthetics.
 |
||
| Scheme 8 Reagents and conditions: (a) (i) HCl,PhCH3, r.t., 1 h; (ii) PhCH3, SOCl2, 55 °C, 1 h; (b) substituted anilines, 58 °C, 5 h; and (c) (i) RBr, K2CO3, DMF, 80 °C, 10 h; (ii) HCl, r.t., 5 min. | ||
SYN
Prepn: A. F. Thuresson, C. Bovin, WO 8500599 (1985 to Apothekernes); H.-J. Federsel et al., Acta Chem. Scand. B41, 757 (1987).

CLIP
Ropivacaine hydrochloride was synthesized from L-2-pipecolic acid by successive reaction with SOCl2 and 2,6-dimethylaniline at 40 °C under ultrasonic irradiation to yield L-N-(2,6-dimethylphenyl)piperidin-2-carboxamide (4), and 4 was reacted with 1-bromopropane at 50 °C for 1 h under ultrasonic irradiation. The effects of reaction solvent, temperature and time under ultrasonic irradiation were investigated. Compared with conventional methods, present procedures have the advantages in milder conditions, shorter reaction time and higher yields. The total yield was 67.5%, [α]25 D= – 6.6°(c = 2, H2O).

SYN
Ropivacaine (3.1.37) (Naropin) is the pure S(–)-enantiomer of propivacaine released for clinical use in 1996. It is a long-acting, well tolerated local anesthetic agent and first produced as a pure enantiomer. Its effects and mechanism of action are similar to other local anesthetics working via reversible inhibition of sodium ion influx in nerve fibers. It may be a preferred option among other drugs among this class of compounds because of its reduced CNS and cardiotoxic potential and its lower propensity for motor block in the management of postoperative pain and labor pain [48–58].
The synthesis of ropivacaine (3.1.37) was carried out starting with l-pipecolic acid (3.1.34), prepared by a resolution of (±)-pipecolic acid with (+)-tartaric acid, which was dissolved in acetyl chloride and converted to acid chloride (3.1.35) with phosphorus pentachloride. The obtained compound (3.1.35) dissolved in toluene a solution of 2,6-xylidine (3.1.28) dissolved in the mixture of equal volumes of acetone, and N-methyl-2-pyrrolidone was added at 70°C to give (+)-l-pipecolic acid-2,6-xylidide (3.1.36). Reaction of this compound with propyl bromide in presence of potassium carbonate in i-PrOH/H2O gave the desired ropivacaine (3.1.37) [59] (Scheme 3.6).


Scheme 3.6. Synthesis of ropivacaine.
Another approach for the synthesis of ropivacaine (3.1.37) was proposed via a resolution of enantiomers of chiral pipecolic acid-2,6-xylidide [60].
SYN

Scheme 21. Generation of ‘cation pool’ and its applications.
Reproduced from Yoshida, J.; Suga, S.; Suzuki, S.; et al. J. Am. Chem. Soc. 1999, 121, 9546–9549, and Shankaraiah, N.; Pilli, R. A.; Santos, L. S. Tetrahedron Lett. 2008, 49, 5098–5100.
CLIP
Process R&D under the magnifying glass: Organization, business model, challenges, and scientific context
Hans-Jürgen Federsel, in Bioorganic & Medicinal Chemistry, 2010
The synthesis of ropivacaine is achieved in only three steps, as in the previous example, comprised of a resolution of a racemic, commercially available starting material (pipecoloxylidide) followed by an N-alkylation and the final precipitation of the product as its HCl salt.14,24 Focusing on the middle step—the attachment of a propyl moiety onto the piperidine nitrogen—this reaction when developed in the laboratory and scaled up to maximum pilot plant volume (1000 L) behaved very well (Scheme 3). Thus, boiling the reaction mixture (reactants in a H2O/organic solvent mixture in the presence of a solid inorganic base) for an extended period of time (6 h) at high temperature (100 °C), the transformation was considered complete once a sample of the process solution showed <1% of remaining starting material. In preparation for launch, the method that had been thoroughly investigated and tested over a number of years and proven reliable on scale up had to be validated in the authentic 4000 L production equipment. Much to our surprise (and shock) we, however, found that the reaction came to a complete stand still long before reaching the expected end point. With a large amount of un-reacted starting material (30–40%) we were facing a situation that had never occurred during the lengthy development phase and this put the whole project in a very critical state as we were not able to reproduce the manufacturing method.





join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
History
Ropivacaine was developed after bupivacaine was noted to be associated with cardiac arrest, particularly in pregnant women. Ropivacaine was found to have less cardiotoxicity than bupivacaine in animal models.
Clinical use
Contraindications
Ropivacaine is contraindicated for intravenous regional anaesthesia (IVRA). However, new data suggested both ropivacaine (1.2-1.8 mg/kg in 40ml) and levobupivacaine (40 ml of 0.125% solution) be used, because they have less cardiovascular and central nervous system toxicity than racemic bupivacaine.[1]
Adverse effects
Adverse drug reactions (ADRs) are rare when it is administered correctly. Most ADRs relate to administration technique (resulting in systemic exposure) or pharmacological effects of anesthesia, however allergic reactions can rarely occur.
Systemic exposure to excessive quantities of ropivacaine mainly result in central nervous system (CNS) and cardiovascular effects – CNS effects usually occur at lower blood plasma concentrations and additional cardiovascular effects present at higher concentrations, though cardiovascular collapse may also occur with low concentrations. CNS effects may include CNS excitation (nervousness, tingling around the mouth, tinnitus, tremor, dizziness, blurred vision, seizures followed by depression (drowsiness, loss of consciousness), respiratory depression and apnea). Cardiovascular effects include hypotension, bradycardia, arrhythmias, and/or cardiac arrest – some of which may be due to hypoxemia secondary to respiratory depression.[2]
Postarthroscopic glenohumeral chondrolysis
Ropivacaine is toxic to cartilage and their intra-articular infusions can lead to Postarthroscopic glenohumeral chondrolysis.[3]
Treatment of overdose
As for bupivacaine, Celepid, a commonly available intravenous lipid emulsion, can be effective in treating severe cardiotoxicity secondary to local anaesthetic overdose in animal experiments[4] and in humans in a process called lipid rescue.[5][6][7]
References
- ^ (Basic of Anesthesia, Robert Stoelting, page 289)
- ^ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ^ Gulihar A, Robati S, Twaij H, Salih A, Taylor GJ (December 2015). “Articular cartilage and local anaesthetic: A systematic review of the current literature”. Journal of Orthopaedics. 12 (Suppl 2): S200-10. doi:10.1016/j.jor.2015.10.005. PMC 4796530. PMID 27047224.
- ^ Weinberg G, Ripper R, Feinstein DL, Hoffman W (2003). “Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity”. Regional Anesthesia and Pain Medicine. 28 (3): 198–202. doi:10.1053/rapm.2003.50041. PMID 12772136. S2CID 6247454.
- ^ Picard J, Meek T (February 2006). “Lipid emulsion to treat overdose of local anaesthetic: the gift of the glob”. Anaesthesia. 61 (2): 107–9. doi:10.1111/j.1365-2044.2005.04494.x. PMID 16430560. S2CID 29843241.
- ^ Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB (July 2006). “Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest”. Anesthesiology. 105 (1): 217–8. doi:10.1097/00000542-200607000-00033. PMID 16810015.
- ^ Litz RJ, Popp M, Stehr SN, Koch T (August 2006). “Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion”. Anaesthesia. 61 (8): 800–1. doi:10.1111/j.1365-2044.2006.04740.x. PMID 16867094. S2CID 43125067.
External links
- “Ropivacaine”. Drug Information Portal. U.S. National Library of Medicine.
- “Ropivacaine hydrochloride”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Naropin |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87%–98% (epidural) |
| Metabolism | Liver (CYP1A2-mediated) |
| Elimination half-life | 1.6–6 hours (varies with administration route) |
| Excretion | Kidney 86% |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.128.244 |
| Chemical and physical data | |
| Formula | C17H26N2O |
| Molar mass | 274.408 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 144 to 146 °C (291 to 295 °F) |
| (verify) | |
Patent
Non-Patent
/////////////Ropivacaine, Anesthetic, ропивакаин , روبيفاكائين , 罗哌卡因 , DRopivacaine Hydrochloride Hydrate, LEA-103, NA-001, (-)-LEA-103
CCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C