

Esreboxetine succinate
Esreboxetine is a selective norepinephrine reuptake inhibitor which was under development by Pfizer for the treatment of neuropathic pain and fibromyalgia but failed to show significant benefit over currently available medications and was discontinued.[1][2][3][4] It is the (S,S)-(+)-enantiomer of reboxetine and is even more selective in comparison.[1][5]
However, recently it has been shown that esreboxetine could be effective in fibromyalgia patients.[6]

Reboxetine mesylate (1) and succinate (2).

CLIP
http://pubs.rsc.org/en/Content/ArticleHtml/2012/GC/c1gc15921f
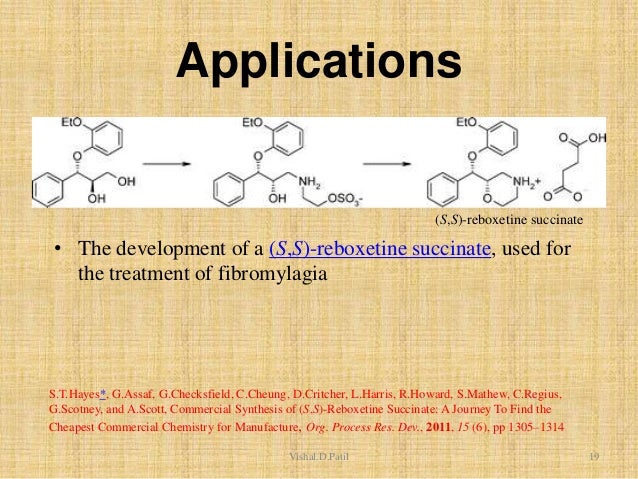
| Scheme 1 The synthesis of (±)-reboxetine mesylate,4 the Active Pharmaceutical Ingredient (API) for Edronax™. |
 |
||
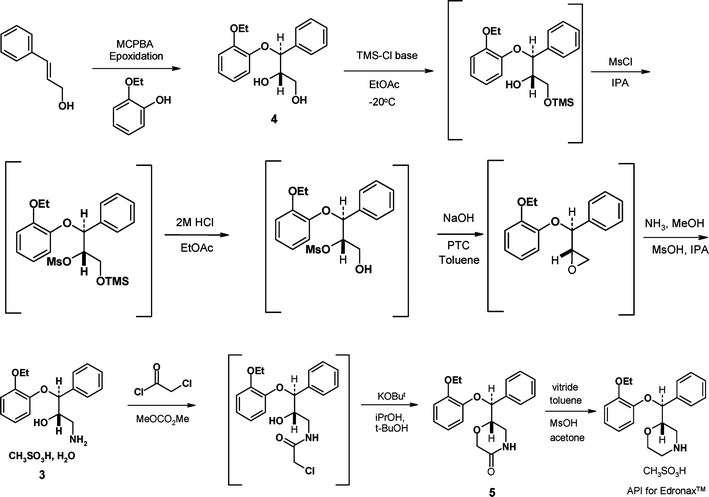
| Scheme 2 The conversion of (±)-reboxetine mesylate to (S,S)-reboxetine succinate. | ||
 |
||
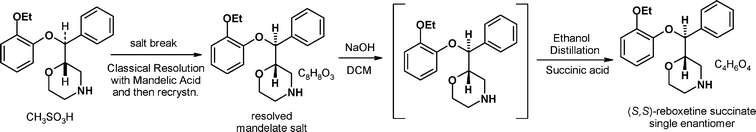
| Scheme 3 The Pfizer early resolution route to (S,S)-reboxetine succinate. | ||
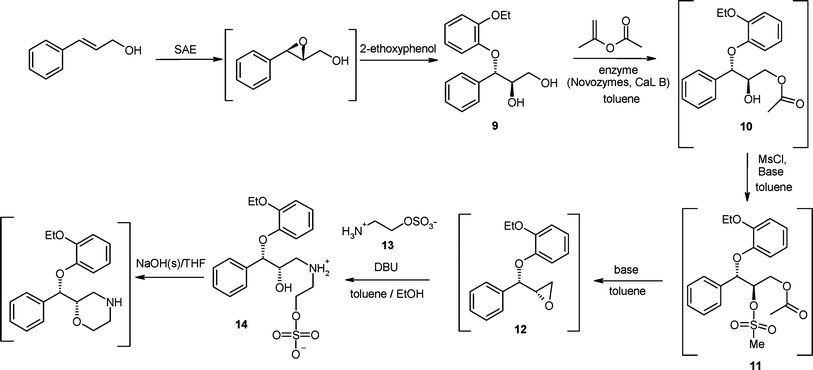
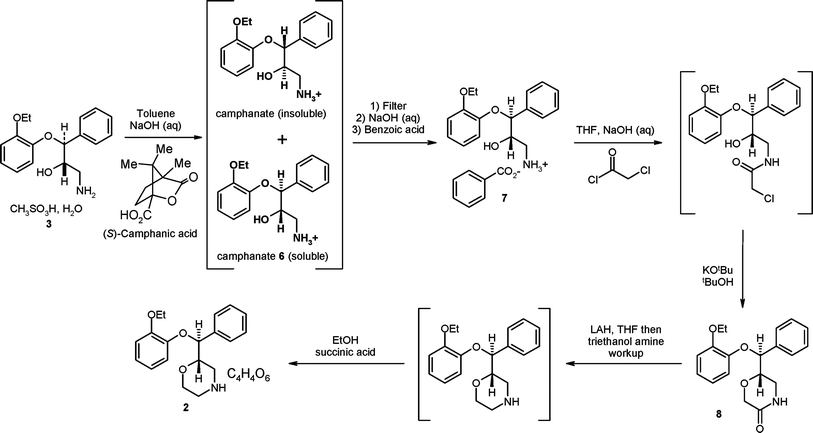
| Scheme 4 The Pfizer asymmetric synthesis for (S,S)-reboxetine intended for commercialisation. |
CLIP
(S,S)-Reboxetine succinate (3) (Figure 1) has been under late-stage development at Pfizer for the medication of neuropathic and fibromyalgia pain.(16)
16.(a) Hughes, B.; McKenzie, I.; Stoker, M. J. WO2006/000903, May 1, 2006.
(b) Allen, A. J.; Hemrick-Luecke, S.; Sumner, C. R.; Wallace, O. B. WO2005/060949, July 7, 2005.
(c) Kelsey, D. K. WO2005/021095, Oct 3, 2005.
(d) Allen, A. J.; Kelsey, D. K. WO 2005/020976, Oct 3, 2005.
(e) Sumner, C. R. WO2005/020975, Oct 3, 2005.
(f) Hassan, F. WO2004/016272, Feb 26, 2004.
PAPER
Process Development for (S,S)-Reboxetine Succinate via a Sharpless Asymmetric Epoxidation
http://pubs.acs.org/doi/abs/10.1021/op700007g?crel=US_AC_eAdv_Blog
Abstract

Reboxetine mesylate is a selective norepinephrine uptake inhibitor (NRI) currently marketed as the racemate. The (S,S)-enantiomer of reboxetine is being evaluated for the treatment of neuropathic pain and a variety of other indications. (S,S)-Reboxetine has usually been prepared by resolution of the racemate as the (−)-mandelate salt, an inherently inefficient process. A chiral synthesis starting with a Sharpless asymmetric epoxidation of cinnamyl alcohol to yield (R,R)-phenylglycidol was developed. (R,R)-Phenylglycidol was reacted without isolation with 2-ethoxyphenol to give 4, which was isolated by direct crystallization. Key process variables for the asymmetric epoxidation were investigated. Conversion of (R,S)-4 to reboxetine parallels the racemic synthesis with streamlined and optimized processing conditions. (S,S)-Reboxetine free base was converted directly to the succinate salt without isolation as the mesylate salt.
(2S,3S)-Reboxetine Succinate (9).
mp 145.2−147.1 °C (lit. mp 148 °C).8 1H NMR (400.13 MHz, CDCl3) δ 1.41 (t, J = 7.0 Hz, 3H), 2.4 (s, 4H), 2.9−3.06 (m, 2H), 3.15−3.22 (m, 2H), 3.81−3.86 (m, 1H), 4.02−4.09 (m, 3H), 4.17−4.24 (m, 1H), 5.13 (d, J = 4.3 Hz), 6.66−6.90 (m, 4H), 7.26−7.39 (m, 5H). 13C NMR (100.62 MHz, CDCl3) δ 15.08, 31.89, 43.24, 44.84, 64.72, 76.91, 82.91, 113.94, 118.27, 121.1, 127.38, 128.66, 136.94, 149.8, 178.73. LRMS-APCI m/z calcd for C19H23NO3 (M + H)+: 314. Found: m/z = 314 [M + 1]+. Anal. Calcd for C19H23NO3−C4H6O4: C, 64.02; H, 6.77; N, 3.25. Found: C, 63.99; H, 6.77; N, 3.16. [α]32.4D +17.24° (c 0.5, EtOH).
8) Zampieri, M.; Airoldi, A.; Martini, A. WO2003/106441, 12/24/03.
PAPER
Commercial Synthesis of (S,S)-Reboxetine Succinate: A Journey To Find the Cheapest Commercial Chemistry for Manufacture
http://pubs.acs.org/doi/abs/10.1021/op200181f
Abstract

The development of a synthetic process for (S,S)-reboxetine succinate, a candidate for the treatment of fibromylagia, is disclosed from initial scale-up to deliver material for registrational stability testing through to commercial route evaluation and subsequent nomination. This entailed evaluation of several alternative routes to result in what would have been a commercially attractive process for launch of the compound.
(2S,3S)-2-[α-(2-Ethoxyphenoxy)benzyl]morpholine Succinate Salt (S,S)-Reboxetine Succinate
(S,S)-reboxetine succinate (897 g, 82%) as a white solid. 1H NMR (400 MHz, d6-DMSO) δ 7.22–7.54 (m, 5H), 6.66–6.96 (m, 4H), 5.27 (d, J = 6.0 Hz, 1H), 4.01 (q, J = 7.1 Hz, 2H), 3.83 (m, 2H), 3.50 (m, 2H), 2.61–2.82 (m, 3H), 2.34 (br s, 4H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, d6-DMSO) δ 174.4, 149.0, 147.3, 137.8, 128.2, 127.3, 120.7, 116.7, 114.4, 80.8, 77.5, 65.9, 64.1, 45.8, 44.1, 39.7, 39.
References[edit]
- ^ Jump up to:a b Matilda Bingham; Napier, Susan Jolliffe (2009). Transporters as Targets for Drugs (Topics in Medicinal Chemistry). Berlin: Springer. ISBN 3-540-87911-0.
- Jump up^ Rao SG (October 2009). “Current progress in the pharmacological therapy of fibromyalgia”. Expert Opinion on Investigational Drugs. 18 (10): 1479–93. PMID 19732029. doi:10.1517/13543780903203771.
- Jump up^ “Search of: esreboxetine – List Results – ClinicalTrials.gov”.
- Jump up^ “Musculoskeletal Report: Pfizer Stops Work on Esreboxetine for FM”.
- Jump up^ Fish, P. V.; MacKenny, M.; Bish, G.; Buxton, T.; Cave, R.; Drouard, D.; Hoople, D.; Jessiman, A.; Miller, D.; Pasquinet, C.; Patel, B.; Reeves, K.; Ryckmans, T.; Skerten, M.; Wakenhut, F. (2009). “Enantioselective synthesis of (R)- and (S)-N-Boc-morpholine-2-carboxylic acids by enzyme-catalyzed kinetic resolution: application to the synthesis of reboxetine analogs”. Tetrahedron Letters. 50 (4): 389. doi:10.1016/j.tetlet.2008.11.025.
- Jump up^ Arnold, L. M., Hirsch, I., Sanders, P., Ellis, A. and Hughes, B. (2012), Safety and efficacy of esreboxetine in patients with fibromyalgia: A fourteen-week, randomized,
REFERENCES
1: Fujimori I, Yukawa T, Kamei T, Nakada Y, Sakauchi N, Yamada M, Ohba Y, Takiguchi M, Kuno M, Kamo I, Nakagawa H, Hamada T, Igari T, Okuda T, Yamamoto S, Tsukamoto T, Ishichi Y, Ueno H. Design, synthesis and biological evaluation of a novel series of peripheral-selective noradrenaline reuptake inhibitor. Bioorg Med Chem. 2015 Aug 1;23(15):5000-14. doi: 10.1016/j.bmc.2015.05.017. Epub 2015 May 15. PubMed PMID: 26051602.
2: Shen F, Tsuruda PR, Smith JA, Obedencio GP, Martin WJ. Relative contributions of norepinephrine and serotonin transporters to antinociceptive synergy between monoamine reuptake inhibitors and morphine in the rat formalin model. PLoS One. 2013 Sep 30;8(9):e74891. doi: 10.1371/journal.pone.0074891. eCollection 2013. PubMed PMID: 24098676; PubMed Central PMCID: PMC3787017.
3: Arnold LM, Hirsch I, Sanders P, Ellis A, Hughes B. Safety and efficacy of esreboxetine in patients with fibromyalgia: a fourteen-week, randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2012 Jul;64(7):2387-97. doi: 10.1002/art.34390. PubMed PMID: 22275142.
4: Arnold LM, Chatamra K, Hirsch I, Stoker M. Safety and efficacy of esreboxetine in patients with fibromyalgia: An 8-week, multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2010 Aug;32(9):1618-32. doi: 10.1016/j.clinthera.2010.08.003. PubMed PMID: 20974319.
5: Klarskov N, Scholfield D, Soma K, Darekar A, Mills I, Lose G. Measurement of urethral closure function in women with stress urinary incontinence. J Urol. 2009 Jun;181(6):2628-33; discussion 2633. doi: 10.1016/j.juro.2009.01.114. Epub 2009 Apr 16. PubMed PMID: 19375093.
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C19H23NO3 |
| Molar mass | 313.391 g/mol |
| 3D model (JSmol) | |
////////////(+)-(S,S)-Reboxetine, (S,S)-Reboxetine, Reboxetine, Esreboxetine succinate
CCOc1ccccc1O[C@H]([C@@H]2CNCCO2)c3ccccc3.OC(=O)CCC(=O)O















