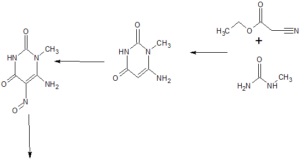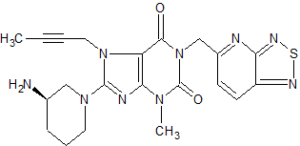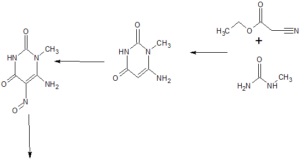PRESENTING 2 MOLECULES………..I AM NOT SURE WHICH IS TITLE MOLECULE
EMAIL ME amcrasto@gmail.com
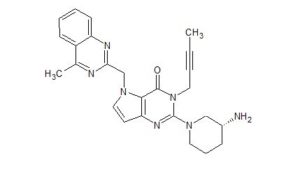
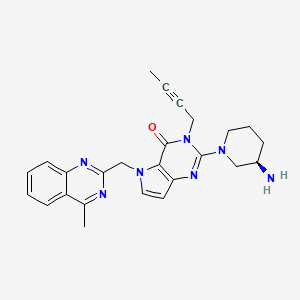
| Molecular Formula: |
C25H27N7O |
| Molecular Weight: |
441.539 g/mol |
2-[(3R)-3alpha-Aminopiperidino]-3-(2-butynyl)-5-(4-methyl-2-quinazolinylmethyl)-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidine-4-one
CAS 1428445-40-2
REF Bioorganic & Medicinal Chemistry (2013), 21(7), 1749-1755.
NEXT ONE………………

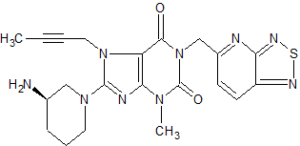
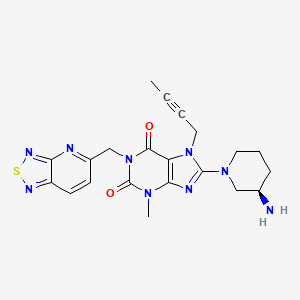
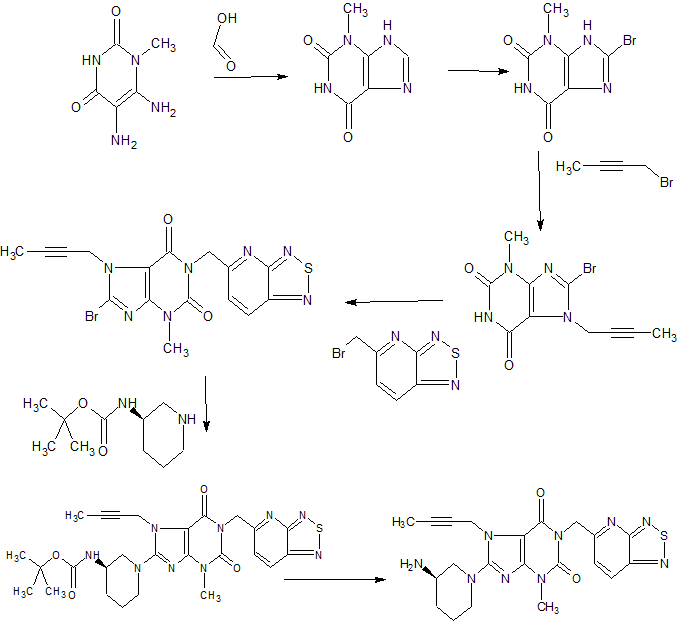
CAS 1415912-31-0
1H-Purine-2,6-dione, 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-([1,2,5]thiadiazolo[3,4-b]pyridin-5-ylmethyl)-
8-[(3R)-3-Amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-([1,2,5]thiadiazolo[3,4-b]pyridin-5-ylmethyl)-1H-purine-2,6-dione
8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-([1,2,5]thiadiazolo[3,4-b]pyridin-5-ylmethyl)purine-2,6-dione
| Molecular Formula: |
C21H23N9O2S |
| Molecular Weight: |
465.536 g/mol |
REF CN 102807568, CN 105315301, WO 2016019868
Salt………..

CAS 1874255-95-4
TILOGLIPTIN
HWH-ZGC-2-143
Guangzhou Institutes of Biomedicine and Health

Chia Tai Tianqing Pharmaceutical Group Co Ltd;

Non-insulin dependent diabetes
Dipeptidyl peptidase IV inhibitor (oral, type 2 diabetes),
DPP-IV inhibitors (oral, type 2 diabetes), Guangzhou Institutes of Biomedicine and Health/Jiangsu Chia Tai Tianqing Pharmaceutical ; HWH-ZGC-2-143 ;
Novel polymorphic forms of thiadiazole derivatives, preferably aglucin, sitagliptin, saxagliptin, vildagliptin, levaratine, useful for treating type II diabetes. Guangzhou Institutes of Biomedicine and Health , in collaboration with Jiangsu Chia Tai Tianqing Pharmaceutical , is investigating tilogliptin , an oral dipeptidyl peptidase IV inhibitor and a pyrrolopyrimidine analog, for treating type 2 diabetes.
As of June 2017, Centaurus BioPharma is developing diabetes therapy, CT-1006 and CT-1005 (in preclinical development) for treating diabetes mellitus.
See WO2016019868, claiming novel citric acid salt of 8-((R)-3-amino-piperidin-1-yl)-1-([1,2,5]-thiadiazolo [3,4-b] pyridine-5methyl)-7-(2-butyn-1-yl)-3-methyl-xanthine, coassigned to Lianyungang Runzhong Pharmaceutical .

PATENT
WO2016019868
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016019868
Chinese Patent Application CN102807568 discloses the use of thiadiazole derivatives DPP-IV inhibitors and their use in the treatment and / or prophylaxis of diseases susceptible to DPP-IV inhibition, particularly in the treatment of type II diabetes. There is still a need for a good thiadiazole derivative DPP-IV inhibitor and a pharmaceutically acceptable salt thereof having good pharmacological and bioavailability.
The contents of the invention
In one aspect, the present application provides 8 – ((R) -3-amino-piperidin-1-yl) -1 – ([1,2,5] thiadiazolo [3,4-b] pyridine- Methyl) -7- (2-butyn-1-yl) -3-methyl-xanthine (having the structure of the following formula I, hereinafter referred to as the compound of formula I).
In another aspect, the present application provides monocarbamates of the compounds of formula I wherein the structural formula is as follows:
PATENT
WO 2017088790
Centaurus BioPharma Co Ltd; Chia Tai Tianqing Pharmaceutical Group Co Ltd

DPP-IV (dipeptidyl peptidase IV) is a serine protease that is expressed in various tissues (eg, liver, lung, intestine, kidney, etc.) in vivo, responsible for endogenous peptides (GLP-1 (7 -36)) metabolic cleavage. However, GLP-1 (7-36) has a variety of beneficial effects in the body, including stimulation of insulin secretion, inhibition of glucagon secretion, promotion of fullness and delayed gastric emptying. Thus, inhibition of DPP-IV can be used to prevent and / or treat diabetes, particularly type II diabetes. There are a variety of DPP-IV inhibitors listed, such as aglucin, sitagliptin, saxagliptin, vildagliptin, levaratine and so on.
Chinese Patent Application CN102807568 discloses a thiadiazole derivative DPP-IV inhibitor as shown in Formula I or Formula II wherein said compound of formula (especially compound 7) has a very good DPP-IV inhibitory activity. In addition, compound 7 also has a very good in vivo metabolic level and a very suitable in vivo half-life, particularly suitable as a DPP-IV inhibitor drug.
In addition to the therapeutic efficacy, the drug developer attempts to provide a suitable form of the active molecule having properties as a drug (e.g., processing, preparation, storage stability, etc.). Therefore, the discovery of the form of the desired nature of the drug development is also essential.
To a 30 L glass autoclave was added 5.5 L of ethanol, 550 g of an intermediate of 1,256 g of (R) 3-aminopiperidine dihydrochloride, 414 g of sodium bicarbonate, stirred and heated to a temperature of 75 ° C to 80 ° C ℃, stirring reaction 4h. TLC (254 nm UV light, methanol: dichloromethane: aqueous ammonia = 1: 10: 0.1, Rf intermediate 1 = 0.7, Rf product = 0.5) was monitored until intermediate 1 was complete, filtered, and ethanol washed. The filtrate 45 ± 5 ℃ under reduced pressure evaporated, add 5L of methylene chloride dissolved, 5L purified water washing; add 5L purified water, 288g citric acid extraction, 2.5L purified water extraction organic phase, combined with water; Methyl chloride and 10L ethanol; add 5L dichloromethane, the temperature control does not exceed 30 degrees, slowly adding sodium hydroxide solution, extraction and separation; organic phase washed with 5L purified water; anhydrous sodium sulfate drying organic phase. Filtered and the filtrate 30 ± 5 ° C evaporated to dryness under reduced pressure to give 372 g of the compound of formula 7 as an amorphous form
Paper
Discovery of potent dipeptidyl peptidase IV inhibitors through pharmacophore hybridization and hit-to-lead optimization
- Shaogao Zenga, ,
- Hui Xiea, ,
- Li-li Zenga,
- Xin Lua,
- Xin Zhaoa,
- Gui-cheng Zhanga,
- Zheng-chao Tua,
- Hong-jiang Xub,
- Ling Yangb,
- Xi-quan Zhangb,
- Wenhui Hua, c, ,
- a Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Science, 190 Kaiyuan Avenue, Guangzhou Science Park, Guangzhou 510530, China
- b Jiangsu Chia-Tai Tianqing Pharmaceutical Co. Ltd, No. 8 Julong North Rd., Xinpu Lianyungang, Jiangsu 222006, China
- c State Key Laboratory of Respiratory Disease, Guangzhou 510120, China
- https://doi.org/10.1016/j.bmc.2013.01.062

- A novel dipeptidyl peptidase IV inhibitor hit (5, IC50 = 0.86 μM) was structurally derived from our recently disclosed preclinical candidate 4 by replacing the cyanobenzyl with a butynyl based on pharmacophore hybridization. A hit-to-lead optimization effort was then initiated to improve its potency. Most N-substituted analogs exhibited good in vitro activity, and compound 18o (IC50 = 1.55 nM) was identified to be a potent dipeptidyl peptidase IV inhibitor with a significantly improved pharmacokinetic properties (bioavailablity: 41% vs 82.9%; T1/2: 2 h vs 4.9 h).
//////TILOGLIPTIN
N[C@@H]1CCCN(C1)C5=Nc4ccn(Cc3nc2ccccc2c(C)n3)c4C(=O)N5CC#CC
N[C@@H]1CCCN(C1)c5nc4c(C(=O)N(Cc2ccc3nsnc3n2)C(=O)N4C)n5CC#CC