SEQUENCE
Sequence Modifications
| Type | Location | Description |
|---|---|---|
| modified base | g-1 | m7g |
| modified base | g-1 | 3′-me |
| modified base | a-2 | am |
| uncommon link | g-1 – a-2 | 5′->5′ triphosphate |
Tozinameran
Pfizer–BioNTech COVID-19 vaccine
トジナメラン (JAN);
コロナウイルス修飾ウリジンRNAワクチン;
RNA (recombinant 5′-[1,2-[(3′-O-methyl)m7G-(5’→5′)-ppp-Am]]-capped all uridine→N1-methylpseudouridine-substituted severe acute respiratory syndrome coronavirus 2 secretory signal peptide contg. spike glycoprotein S1S2-specifying plus 5′- and 3′-untranslated flanking region-contg. poly(A)-tailed messenger BNT162b2), inner salt
Nucleic Acid Sequence
Sequence Length: 42841106 a 1315 c 1062 g 801 umodified
APPROVED JAPAN Comirnaty, 2021/2/14
CAS 2417899-77-3
|
Active immunization (SARS-CoV-2)
|
Tozinameran is mRNA encoding full length of spike protein analog of SARS-CoV-2
Target Severe acute respiratory syndrome coronavirus 2 spike glycoprotein
Coronavirus disease – COVID-19
| FORM | ROUTE | STRENGTH |
|---|---|---|
| Injection, suspension | Intramuscular | 0.23 mg/1.8mL |
| Suspension | Intramuscular | 30 mcg |
| NAME | INGREDIENTS | DOSAGE | ROUTE | LABELLER | MARKETING START | MARKETING END | ||
|---|---|---|---|---|---|---|---|---|
| Pfizer-BioNTech Covid-19 Vaccine | Pfizer-BioNTech Covid-19 Vaccine (0.23 mg/1.8mL) | Injection, suspension | Intramuscular | Pfizer Manufacturing Belgium NV | 2020-12-12 | Not applicable |
| NAME | DOSAGE | STRENGTH | ROUTE | LABELLER | MARKETING START | MARKETING END | ||
|---|---|---|---|---|---|---|---|---|
| Comirnaty | 30 mcg | Intramuscular | Bio N Tech Manufacturing Gmb H | 2021-01-06 | Not applicable | |||
| Pfizer-BioNTech Covid-19 Vaccine | Suspension | 30 mcg | Intramuscular | Biontech Manufacturing Gmbh | 2020-12-14 | Not applicable | ||
| Pfizer-BioNTech Covid-19 Vaccine | Injection, suspension | 0.23 mg/1.8mL | Intramuscular | Pfizer Manufacturing Belgium NV | 2020-12-12 | Not applicable |
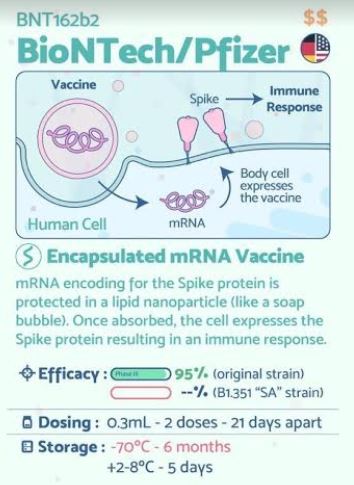
The Pfizer–BioNTech COVID‑19 vaccine (pINN: tozinameran), sold under the brand name Comirnaty,[13] is a COVID-19 vaccine developed by the German company BioNTech in cooperation with Pfizer. It is both the first COVID-19 vaccine to be authorized by a stringent regulatory authority for emergency use[14][15] and the first cleared for regular use.[16]
It is given by intramuscular injection. It is an RNA vaccine composed of nucleoside-modified mRNA (modRNA) encoding a mutated form of the spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles.[17] The vaccination requires two doses given three weeks apart.[18][19][20] Its ability to prevent severe infection in children, pregnant women, or immunocompromised people is unknown, as is the duration of the immune effect it confers.[20][21][22] As of February 2021, it is one of two RNA vaccines being deployed against COVID‑19, the other being the Moderna COVID‑19 vaccine. A third mRNA-based COVID-19 vaccine, CVnCoV, is in late-stage testing.[23]
Trials began in April 2020; by November, the vaccine had been tested on more than 40,000 people.[24] An interim analysis of study data showed a potential efficacy of over 90% in preventing infection within seven days of a second dose.[19][20] The most common side effects include mild to moderate pain at the injection site, fatigue, and headache.[25][26] As of December 2020, reports of serious side effects, such as allergic reactions, have been very rare,[a] and no long-term complications have been reported.[28] Phase III clinical trials are ongoing: monitoring of the primary outcomes will continue until August 2021, while monitoring of the secondary outcomes will continue until January 2023.[18]
In December 2020, the United Kingdom was the first country to authorize the vaccine on an emergency basis,[28] soon followed by the United States, the European Union and several other countries globally.[29][30][6][31][32]
BioNTech is the initial developer of the vaccine, and partnered with Pfizer for development, clinical research, overseeing the clinical trials, logistics, finances and for manufacturing worldwide with the exception of China.[33] The license to distribute and manufacture in China was purchased by Fosun, alongside its investment in BioNTech.[34][35] Distribution in Germany and Turkey is by BioNTech itself.[36] Pfizer indicated in November 2020, that 50 million doses could be available globally by the end of 2020, with about 1.3 billion doses in 2021.[20]
Pfizer has advanced purchase agreements of about US$3 billion for providing a licensed vaccine in the United States, the European Union, the United Kingdom, Japan, Canada, Peru, Singapore, and Mexico.[37][38] Distribution and storage of the vaccine is a logistics challenge because it needs to be stored at temperatures between −80 and −60 °C (−112 and −76 °F),[39] until five days before vaccination[38][39] when it can be stored at 2 to 8 °C (36 to 46 °F), and up to two hours at temperatures up to 25 °C (77 °F)[40][11] or 30 °C (86 °F).[41][42] In February 2021, Pfizer and BioNTech asked the U.S. Food and Drug Administration (FDA) to update the emergency use authorization (EUA) to permit the vaccine to be stored at between −25 and −15 °C (−13 and 5 °F) for up to two weeks before use.[43]
Development and funding
Before COVID-19 vaccines, a vaccine for an infectious disease had never before been produced in less than several years, and no vaccine existed for preventing a coronavirus infection in humans.[44] After the COVID-19 virus was detected in December 2019,[45] the development of BNT162b2 was initiated on 10 January 2020, when the SARS-CoV-2 genetic sequences were released by the Chinese Center for Disease Control and Prevention via GISAID,[46][47][48] triggering an urgent international response to prepare for an outbreak and hasten development of preventive vaccines.[49][50]
In January 2020, German biotech-company BioNTech started its program ‘Project Lightspeed’ to develop a vaccine against the new COVID‑19 virus based on its already established mRNA-technology.[24] Several variants of the vaccine were created in their laboratories in Mainz, and 20 of those were presented to experts of the Paul-Ehrlich-Institute in Langen.[51] Phase I / II Trials were started in Germany on 23 April 2020, and in the U.S. on 4 May 2020, with four vaccine candidates entering clinical testing. The Initial Pivotal Phase II / III Trial with the lead vaccine candidate ‘BNT162b2’ began in July. The Phase III results indicating a 95% effectiveness of the developed vaccine were published on 18 November 2020.[24]
BioNTech received a US$135 million investment from Fosun in March 2020, in exchange for 1.58 million shares in BioNTech and the future development and marketing rights of BNT162b2 in China,[35] Hong Kong, Macau and Taiwan.[52]
In June 2020, BioNTech received €100 million (US$119 million) in financing from the European Commission and European Investment Bank.[53] In September 2020, the German government granted BioNTech €375 million (US$445 million) for its COVID‑19 vaccine development program.[54]
Pfizer CEO Albert Bourla stated that he decided against taking funding from the US government’s Operation Warp Speed for the development of the vaccine “because I wanted to liberate our scientists [from] any bureaucracy that comes with having to give reports and agree how we are going to spend the money in parallel or together, etc.” Pfizer did enter into an agreement with the US for the eventual distribution of the vaccine, as with other countries.[55]
Clinical trials
Preliminary results from Phase I–II clinical trials on BNT162b2, published in October 2020, indicated potential for its efficacy and safety.[17][56] During the same month, the European Medicines Agency (EMA) began a periodic review of BNT162b2.[57]
The study of BNT162b2 is a continuous-phase trial in Phase III as of November 2020.[18] It is a “randomized, placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection, and efficacy study in healthy individuals”.[18] The early-stage research determined the safety and dose level for two vaccine candidates, with the trial expanding during mid-2020 to assess efficacy and safety of BNT162b2 in greater numbers of participants, reaching tens of thousands of people receiving test vaccinations in multiple countries in collaboration with Pfizer and Fosun.[20][35]
The Phase III trial assesses the safety, efficacy, tolerability, and immunogenicity of BNT162b2 at a mid-dose level (two injections separated by 21 days) in three age groups: 12–15 years, 16–55 years or above 55 years.[18] For approval in the EU, an overall vaccine efficacy of 95% was confirmed by the EMA.[58] The EMA clarified that the second dose should be administered three weeks after the first dose.[59]
| Efficacy endpoint | Vaccine efficacy (95% confidence interval) [%] |
|---|---|
| After dose 1 to before dose 2 | 52.4 (29.5, 68.4) |
| ≥10 days after dose 1 to before dose 2 | 86.7 (68.6, 95.4) |
| Dose 2 to 7 days after dose 2 | 90.5 (61.0, 98.9) |
| ≥7 days after dose 2 (subjects without evidence of infection prior to 7 days after dose 2) | |
| Overall | 95.0 (90.0, 97.9) |
| 16–55 years | 95.6 (89.4, 98.6) |
| ≥55 years | 93.7 (80.6, 98.8) |
| ≥65 years | 94.7 (66.7, 99.9) |
The ongoing Phase III trial, which is scheduled to run from 2020 to 2022, is designed to assess the ability of BNT162b2 to prevent severe infection, as well as the duration of immune effect.[20][21][22]
Pfizer and BioNTech started a Phase II/III randomized control trial in healthy pregnant women 18 years of age and older (NCT04754594).[60] The study will evaluate 30 µg of BNT162b2 or placebo administered via intramuscular injection in 2 doses, 21 days apart. The Phase II portion of the study will include approximately 350 pregnant women randomized 1:1 to receive BNT162b2 or placebo at 27 to 34 weeks’ gestation. The Phase III portion of this study will assess the safety, tolerability, and immunogenicity of BNT162b2 or placebo among pregnant women enrolled at 24 to 34 weeks’ gestation. Pfizer and BioNTech announced on 18 February 2021 that the first participants received their first dose in this trial.[61]
Vaccine technology
The BioNTech technology for the BNT162b2 vaccine is based on use of nucleoside-modified mRNA (modRNA) which encodes part of the spike protein found on the surface of the SARS-CoV-2 coronavirus (COVID‑19), triggering an immune response against infection by the virus protein.[62]
The vaccine candidate BNT162b2 was chosen as the most promising among three others with similar technology developed by BioNTech.[18][62][56] Prior to choosing BNT162b2, BioNTech and Pfizer had conducted Phase I trials on BNT162b1 in Germany and the United States, while Fosun performed a Phase I trial in China.[17][63] In these Phase I studies, BNT162b2 was shown to have a better safety profile than the other three BioNTech candidates.[63]
Sequence
The modRNA sequence of the vaccine is 4,284 nucleotides long.[64] It consists of a five-prime cap; a five prime untranslated region derived from the sequence of human alpha globin; a signal peptide (bases 55–102) and two proline substitutions (K986P and V987P, designated “2P”) that cause the spike to adopt a prefusion-stabilized conformation reducing the membrane fusion ability, increasing expression and stimulating neutralizing antibodies;[17][65] a codon-optimized gene of the full-length spike protein of SARS-CoV-2 (bases 103–3879); followed by a three prime untranslated region (bases 3880–4174) combined from AES and mtRNR1 selected for increased protein expression and mRNA stability[66] and a poly(A) tail comprising 30 adenosine residues, a 10-nucleotide linker sequence, and 70 other adenosine residues (bases 4175–4284).[64] The sequence contains no uridine residues; they are replaced by 1-methyl-3′-pseudouridylyl.[64]
Composition
In addition to the mRNA molecule, the vaccine contains the following inactive ingredients (excipients):[67][68][8]
- ALC-0315, ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate)
- ALC-0159, 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide
- 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)
- cholesterol
- dibasic sodium phosphate dihydrate
- monobasic potassium phosphate
- potassium chloride
- sodium chloride
- sucrose
- water for injection
The first four of these are lipids. The lipids and modRNA together form nanoparticles. ALC-0159 is a polyethylene glycol conjugate (that is, a PEGylated lipid).[69]
The vaccine is supplied in a multidose vial as “a white to off-white, sterile, preservative-free, frozen suspension for intramuscular injection“.[11][12] It must be thawed to room temperature and diluted with normal saline before administration.[12]
Authorizations
Expedited
The United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) gave the vaccine “rapid temporary regulatory approval to address significant public health issues such as a pandemic” on 2 December 2020, which it is permitted to do under the Medicines Act 1968.[70] It was the first COVID‑19 vaccine to be approved for national use after undergoing large scale trials,[71] and the first mRNA vaccine to be authorized for use in humans.[14][72] The United Kingdom thus became the first Western country to approve a COVID‑19 vaccine for national use,[73] although the decision to fast-track the vaccine was criticised by some experts.[74]
On 8 December 2020, Margaret “Maggie” Keenan, 90, from Fermanagh, became the first person to receive the vaccine.[75] In a notable example of museums documenting the pandemic, the vial and syringe used for that first dose were saved acquired by The Science Museum in London for its permanent collection.[76] By 20 December, 521,594 UK residents had received the vaccine as part of the national vaccination programme. 70% had been to people aged 80 or over.[77]
After the United Kingdom, the following countries expedited processes to approve the Pfizer–BioNTech COVID‑19 vaccine for use: Argentina,[78] Australia,[79] Bahrain,[80] Canada,[7][81] Chile,[82] Costa Rica,[83] Ecuador,[82] Hong Kong,[84] Iraq,[85] Israel,[86] Jordan,[87] Kuwait,[88] Mexico,[89] Oman,[90] Panama,[91] the Philippines,[92] Qatar,[93] Saudi Arabia,[32][94] Singapore,[95][96] the United Arab Emirates,[97] and the United States.[10]
The World Health Organization (WHO) authorized it for emergency use.[98]
In the United States, an emergency use authorization (EUA) is “a mechanism to facilitate the availability and use of medical countermeasures, including vaccines, during public health emergencies, such as the current COVID‑19 pandemic”, according to the FDA.[99] Following an EUA issuance, BioNTech and Pfizer are expected to continue the Phase III clinical trial to finalize safety and efficacy data, leading to application for licensure (approval) of the vaccine in the United States.[99][100][101] The United States Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) approved recommendations for vaccination of those aged 16 years or older.[102][103]
Standard
On 19 December 2020, the Swiss Agency for Therapeutic Products (Swissmedic) approved the Pfizer–BioNTech COVID‑19 vaccine for regular use, two months after receiving the application, stating that the vaccine fully complied with the requirements of safety, efficacy and quality. This is the first authorization under a standard procedure.[1][104] On 23 December, a Lucerne resident, a 90-year-old woman, became the first person to receive the vaccine in Switzerland.[105] This marked the beginning of mass vaccination in continental Europe.[106]
On 21 December 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended granting conditional marketing authorization for the Pfizer–BioNTech COVID‑19 vaccine under the brand name Comirnaty.[2][107][108] The recommendation was accepted by the European Commission the same day.[107][109]
On February 23, 2021, the Brazilian Health Regulatory Agency approved the Pfizer–BioNTech COVID-19 vaccine under its standard marketing authorization procedure. It became the first COVID-19 vaccine to receive definitive registration rather than emergency use authorization in the country.[110]
Adverse effects
The adverse effect profile of the Pfizer–BioNTech COVID‑19 vaccine is similar to that of other adult vaccines.[20] During clinical trials, the side effects deemed very common[a] are (in order of frequency): pain and swelling at the injection site, tiredness, headache, muscle aches, chills, joint pain, and fever.[68] Fever is more common after the second dose.[68] These effects are predictable and to be expected, and it is particularly important that people be aware of this to prevent vaccine hesitancy.[111]
Severe allergic reaction has been observed in approximately 11 cases per million doses of vaccine administered.[112][113] According to a report by the US Centers for Disease Control and Prevention 71% of those allergic reactions happened within 15 minutes of vaccination and mostly (81%) among people with a documented history of allergies or allergic reactions.[112] The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) advised on 9 December 2020, that people who have a history of “significant” allergic reaction should not receive the Pfizer–BioNTech COVID‑19 vaccine.[114][115][116] On 12 December, the Canadian regulator followed suit, noting that: “Both individuals in the U.K. had a history of severe allergic reactions and carried adrenaline auto injectors. They both were treated and have recovered.”[67]
On 28 January 2021, the European Union published a COVID-19 vaccine safety update which found that “the benefits of Comirnaty in preventing COVID‑19 continue to outweigh any risks, and there are no recommended changes regarding the use the vaccine.”[113][117] No new side effects were identified.[113]
Manufacturing
Pfizer and BioNTech are manufacturing the vaccine in their own facilities in the United States and in Europe in a three-stage process. The first stage involves the molecular cloning of DNA plasmids that code for the spike protein by infusing them into Escherichia coli bacteria. In the United States, this stage is conducted at a small pilot plant in Chesterfield, Missouri[118] (near St. Louis). After four days of growth, the bacteria are killed and broken open, and the contents of their cells are purified over a week and a half to recover the desired DNA product. The DNA is stored in tiny bottles and frozen for shipment. Safely and quickly transporting the DNA at this stage is so important that Pfizer has used its company jet and helicopter to assist.[119]
The second stage is being conducted at plants in Andover, Massachusetts[120] in the United States, and in Germany. The DNA is used as a template to build the desired mRNA strands. Once the mRNA has been created and purified, it is frozen in plastic bags about the size of a large shopping bag, of which each can hold up to 5 to 10 million doses. The bags are placed on special racks on trucks which take them to the next plant.[119]
The third stage is being conducted at plants in Portage, Michigan[121] (near Kalamazoo) in the United States, and Puurs in Belgium. This stage involves combining the mRNA with lipid nanoparticles, then filling vials, boxing vials, and freezing them.[119] Croda International subsidiary Avanti Polar Lipids is providing the requisite lipids.[122] As of November 2020, the major bottleneck in the manufacturing process was combining mRNA with lipid nanoparticles.[119]
In February 2021, Pfizer revealed this entire sequence initially took about 110 days on average from start to finish, and that the company was making progress on reducing that number to 60 days.[123] Vaccine manufacturers normally take several years to optimize the process of making a particular vaccine for speed and cost-effectiveness before attempting large-scale production.[123] Due to the urgency presented by the COVID-19 pandemic, Pfizer began production immediately with the process by which the vaccine had been originally formulated in the laboratory, then started to identify ways to safely speed up and scale up that process.[123]
BioNTech announced in September 2020 that it had signed an agreement to acquire from Novartis a manufacturing facility in Marburg, Germany, to expand their vaccine production capacity.[124] Once fully operational, the facility would produce up to 750 million doses per year, or over 60 million doses per month. The site will be the third BioNTech facility in Europe which currently produces the vaccine, while Pfizer operates at least four production sites in the United States and Europe.
Advance orders and logistics
Pfizer indicated in its 9 November press release that 50 million doses could be available by the end of 2020, with about 1.3 billion doses provided globally by 2021.[20] In February 2021, BioNTech announced it would increase production by more than 50% to manufacture two billion doses in 2021.[125]
In July 2020, the vaccine development program Operation Warp Speed placed an advance order of US$1.95 billion with Pfizer to manufacture 100 million doses of a COVID‑19 vaccine for use in the United States if the vaccine was shown to be safe and effective.[34][126][127][128] By mid-December 2020, Pfizer had agreements to supply 300 million doses to the European Union,[129] 120 million doses to Japan,[130] 40 million doses (10 million before 2021) to the United Kingdom,[22] 20 million doses to Canada,[131] an unspecified number of doses to Singapore,[132] and 34.4 million doses to Mexico.[133] Fosun also has agreements to supply 10 million doses to Hong Kong and Macau.[134] The Hong Kong government said it would receive its first batch of one million doses by the first quarter of 2021.[135]
BioNTech and Fosun agreed to supply Mainland China with a batch of 100 million doses in 2021, subject to regulatory approval. The initial supply will be delivered from BioNTech’s production facilities in Germany.[136]
The vaccine is being delivered in vials that, once diluted, contain 2.25 ml of vaccine (0.45 ml frozen plus 1.8ml diluent).[101] According to the vial labels, each vial contains five 0.3 ml doses, however excess vaccine may be used for one, or possibly two, additional doses.[101][137] The use of low dead space syringes to obtain the additional doses is preferable, and partial doses within a vial should be discarded.[101][138] The Italian Medicines Agency officially authorized the use of excess doses remaining within single vials.[139] As of 8 January 2021, each vial contains six doses.[68][140][141][138] In the United States, vials will be counted as five doses when accompanied by regular syringes and as six doses when accompanied by low dead space syringes.[142]
Logistics in developing countries which have preorder agreements with Pfizer—such as Ecuador and Peru—remain unclear.[38] Even high-income countries have limited cold chain capacity for ultracold transport and storage of a vaccine that degrades within five days when thawed, and requires two shots three weeks apart.[38] The vaccine needs to be stored and transported at ultracold temperatures between −80 and −60 °C (−112 and −76 °F),[39][22][38][143][144] much lower than for the similar Moderna vaccine. The head of Indonesia‘s Bio Farma Honesti Basyir stated that purchasing the vaccine is out of the question for the world’s fourth-most populous country, given that it did not have the necessary cold chain capability. Similarly, India’s existing cold chain network can only handle temperatures between 2 and 8 °C (36 and 46 °F), far above the requirements of the vaccine.[145][146]
In January 2021, Pfizer and BioNTech offered to supply 50 million doses of COVID‑19 vaccine for health workers across Africa between March and the end of 2021, at a discounted price of US$10 per dose.[147]
Name
BNT162b2 was the code name during development and testing,[17][148] tozinameran is the proposed international nonproprietary name (pINN),[149] and Comirnaty is the brand name.[1][2] According to BioNTech, the name Comirnaty “represents a combination of the terms COVID‑19, mRNA, community, and immunity.”[150][151]
The vaccine also has the common name “COVID‑19 mRNA vaccine (nucleoside-modified)”[2] and may be distributed in packaging with the name Pfizer–BioNTech COVID‑19 Vaccine.”[152]
How the Pfizer-BioNTech Vaccine Works

The German company BioNTech partnered with Pfizer to develop and test a coronavirus vaccine known as BNT162b2, the generic name tozinameran or the brand name Comirnaty. A clinical trial demonstrated that the vaccine has an efficacy rate of 95 percent in preventing Covid-19.
A Piece of the Coronavirus
The SARS-CoV-2 virus is studded with proteins that it uses to enter human cells. These so-called spike proteins make a tempting target for potential vaccines and treatments.

Spikes
Spike
protein
gene
CORONAVIRUS
Like the Moderna vaccine, the Pfizer-BioNTech vaccine is based on the virus’s genetic instructions for building the spike protein.
mRNA Inside an Oily Shell
The vaccine uses messenger RNA, genetic material that our cells read to make proteins. The molecule — called mRNA for short — is fragile and would be chopped to pieces by our natural enzymes if it were injected directly into the body. To protect their vaccine, Pfizer and BioNTech wrap the mRNA in oily bubbles made of lipid nanoparticles.

Lipid nanoparticles
surrounding mRNA
Because of their fragility, the mRNA molecules will quickly fall apart at room temperature. Pfizer is building special containers with dry ice, thermal sensors and GPS trackers to ensure the vaccines can be transported at –94°F (–70°C) to stay viable.
Entering a Cell
After injection, the vaccine particles bump into cells and fuse to them, releasing mRNA. The cell’s molecules read its sequence and build spike proteins. The mRNA from the vaccine is eventually destroyed by the cell, leaving no permanent trace.

VACCINE
PARTICLES
VACCINATED
CELL
Spike
protein
mRNA
Translating mRNA
Three spike
proteins combine
Spike
Cell
nucleus
Spikes
and protein
fragments
Displaying
spike protein
fragments
Protruding
spikes
Some of the spike proteins form spikes that migrate to the surface of the cell and stick out their tips. The vaccinated cells also break up some of the proteins into fragments, which they present on their surface. These protruding spikes and spike protein fragments can then be recognized by the immune system.
Spotting the Intruder
When a vaccinated cell dies, the debris will contain many spike proteins and protein fragments, which can then be taken up by a type of immune cell called an antigen-presenting cell.

Debris from
a dead cell
Engulfing
a spike
ANTIGEN-
PRESENTING
CELL
Digesting
the proteins
Presenting a
spike protein
fragment
HELPER
T CELL
The cell presents fragments of the spike protein on its surface. When other cells called helper T cells detect these fragments, the helper T cells can raise the alarm and help marshal other immune cells to fight the infection.
Making Antibodies
Other immune cells, called B cells, may bump into the coronavirus spikes on the surface of vaccinated cells, or free-floating spike protein fragments. A few of the B cells may be able to lock onto the spike proteins. If these B cells are then activated by helper T cells, they will start to proliferate and pour out antibodies that target the spike protein.

HELPER
T CELL
Activating
the B cell
Matching
surface proteins
VACCINATED
CELL
B CELL
SECRETED
ANTIBODIES
Stopping the Virus
The antibodies can latch onto coronavirus spikes, mark the virus for destruction and prevent infection by blocking the spikes from attaching to other cells.

ANTIBODIES
VIRUS
Killing Infected Cells
The antigen-presenting cells can also activate another type of immune cell called a killer T cell to seek out and destroy any coronavirus-infected cells that display the spike protein fragments on their surfaces.

ANTIGEN-
PRESENTING
CELL
Presenting a
spike protein
fragment
ACTIVATED
KILLER
T CELL
INFECTED
CELL
Beginning
to kill the
infected cell
Remembering the Virus
The Pfizer-BioNTech vaccine requires two injections, given 21 days apart, to prime the immune system well enough to fight off the coronavirus. But because the vaccine is so new, researchers don’t know how long its protection might last.

First dose, 0.3ml
Second dose, 21 days later
A preliminary study found that the vaccine seems to offer strong protection about 10 days after the first dose, compared with people taking a placebo:

Cumulative incidence of Covid-19 among clinical trial participants 2.5% 2.0 People taking a placebo
1.5 1.0 Second dose First dose People taking the
Pfizer-BioNTech vaccine
0.5
0
1
2
3
4
8
12
16
Weeks after the first dose
It’s possible that in the months after vaccination, the number of antibodies and killer T cells will drop. But the immune system also contains special cells called memory B cells and memory T cells that might retain information about the coronavirus for years or even decades.
For more about the vaccine, see Pfizer’s Covid Vaccine: 11 Things You Need to Know.
Preparation and Injection
Each vial of the vaccine contains 5 doses of 0.3 milliliters. The vaccine must be thawed before injection and diluted with saline. After dilution the vial must be used within six hours.

References
- ^ Jump up to:a b According to the British National Formulary and MedDRA conventions, side effects are “very common” when they occur in more than 1 in 10 instances; “common”, 1 in 100 to 1 in 10; “uncommon”, 1 in 1,000 to 1 in 100; “rare”, 1 in 10,000 to 1 in 1,000; and “very rare” when they occur in less than 1 in 10,000 instances.[27]
- ^ Jump up to:a b c d e “Comirnaty EPAR”. European Medicines Agency (EMA). Retrieved 23 December 2020.
- ^ “Comirnaty”. Therapeutic Goods Administration (TGA). Retrieved 25 January 2021.
- ^ “Comirnaty (BNT162b2 [mRNA]) COVID‑19 Vaccine Product Information” (PDF). Therapeutic Goods Administration (TGA). Retrieved 25 January 2021.
- ^ Australian Public Assessment Report for BNT162b2 (mRNA) (PDF) (Report). Therapeutic Goods Administration (TGA). Retrieved 25 January 2021.
- ^ Jump up to:a b “Regulatory Decision Summary – Pfizer-BioNTech COVID-19 Vaccine”. Health Canada. 9 December 2020. Archived from the original on 9 December 2020. Retrieved 9 December 2020.
- ^ Jump up to:a b “Pfizer-BioNTech COVID-19 Vaccine (tozinameran)”. Health Canada. Retrieved 15 December 2020.
- ^ Jump up to:a b “Information for Healthcare Professionals on Pfizer/BioNTech COVID-19 vaccine”. Medicines and Healthcare products Regulatory Agency (MHRA). 10 December 2020. Retrieved 21 December 2020.
- ^ “Conditions of Authorisation for Pfizer/BioNTech COVID-19 vaccine”. Medicines and Healthcare products Regulatory Agency (MHRA). 31 December 2020. Retrieved 8 January2021.
- ^ Jump up to:a b “FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine” (Press release). U.S. Food and Drug Administration (FDA). 11 December 2020. Retrieved 11 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b c “Pfizer-BioNTech COVID-19 Vaccine- rna ingredient bnt-162b2 injection, suspension”. DailyMed. Retrieved 14 December 2020.
- ^ Jump up to:a b c Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Review Memorandum (PDF). U.S. Food and Drug Administration (FDA) (Report). 14 December 2020. Retrieved 14 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “Comirnaty EPAR”. European Medicines Agency (EMA). Retrieved 23 December 2020.
- ^ Jump up to:a b “UK medicines regulator gives approval for first UK COVID-19 vaccine” (Press release). Medicines and Healthcare products Regulatory Agency (MHRA). 2 December 2020. Retrieved 2 December 2020.
- ^ Boseley S, Halliday J (2 December 2020). “UK approves Pfizer/BioNTech Covid vaccine for rollout next week”. The Guardian. Retrieved 14 December 2020.
- ^ Jump up to:a b c d e Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. (October 2020). “Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates”. The New England Journal of Medicine. 383 (25): 2439–50. doi:10.1056/NEJMoa2027906. PMC 7583697. PMID 33053279.
- ^ Jump up to:a b c d e f Clinical trial number NCT04368728 for “NCT04368728: Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals” at ClinicalTrials.gov
- ^ Jump up to:a b Palca J (9 November 2020). “Pfizer says experimental COVID-19 vaccine is more than 90% effective”. NPR. Archived from the original on 9 November 2020. Retrieved 9 November 2020.
- ^ Jump up to:a b c d e f g h Herper M (9 November 2020). “Covid-19 vaccine from Pfizer and BioNTech is strongly effective, early data from large trial indicate”. STAT. Archived from the original on 9 November 2020. Retrieved 9 November 2020.
- ^ Jump up to:a b Edwards E (9 November 2020). “Pfizer’s Covid-19 vaccine promising, but many questions remain”. NBC News. Archived from the original on 22 November 2020. Retrieved 12 November 2020.
- ^ Jump up to:a b c d Gallagher J (9 November 2020). “Covid vaccine: First ‘milestone’ vaccine offers 90% protection”. BBC News. Archived from the original on 26 November 2020. Retrieved 9 November 2020.
- ^ “CureVac Initiates Rolling Submission With European Medicines Agency for COVID-19 Vaccine Candidate, CVnCoV”. CureVac (Press release).
- ^ Jump up to:a b c “Update on our COVID-19 vaccine development program with BNT162b2” (PDF)(Press release). BioNTech. 2 December 2020. Retrieved 12 December 2020.
- ^ Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. (December 2020). “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine”. N Engl J Med. 383 (27): 2603–2615. doi:10.1056/NEJMoa2034577. PMC 7745181. PMID 33301246.
- ^ “Questions and Answers About Pfizer-BioNTech COVID-19 Vaccine”. Pfizer. Retrieved 16 December 2020.
- ^ “Adverse reactions to drugs”. British National Formulary. Retrieved 19 December 2020.
- ^ Jump up to:a b “Coronavirus vaccine”. National Health Service. 7 December 2020. Archived from the original on 7 December 2020. Retrieved 7 December 2020.
- ^ Commissioner, Office of the (3 February 2021). “Pfizer-BioNTech COVID-19 Vaccine”. FDA.
- ^ “EMA recommends first COVID-19 vaccine for authorisation in the EU”. European Medicines Agency.
- ^ “Bahrain becomes second country to approve Pfizer COVID-19 vaccine”. Al Jazeera. Archived from the original on 4 December 2020. Retrieved 5 December 2020.
* “Coronavirus: Saudi Arabia approves Pfizer COVID-19 vaccine for use”. Al Arabiya English. 10 December 2020. Archived from the original on 11 December 2020. Retrieved 10 December 2020.
* Solomon DB, Torres N (11 December 2020). “Mexico approves emergency use of Pfizer’s COVID-19 vaccine”. Reuters. Retrieved 12 December 2020.
* Thomas K (20 November 2020). “F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away”. The New York Times. Archived from the original on 12 December 2020. Retrieved 11 December 2020.
* “First shipments of Pfizer-BioNTech vaccine in Singapore by end-Dec; enough vaccines for all by Q3 2021”. The Straits Times. 14 December 2020. Retrieved 14 December 2020. - ^ Jump up to:a b Al Mulla Y (13 December 2020). “Kuwait approves emergency use of Pfizer vaccine”. Gulf News. Retrieved 14 December 2020.
- ^ Browne R (11 November 2020). “What you need to know about BioNTech – the European company behind Pfizer’s Covid-19 vaccine”. CNBC. Retrieved 14 January 2021.
- ^ Jump up to:a b Thomas K, Gelles D, Zimmer C (9 November 2020). “Pfizer’s early data shows vaccine is more than 90% effective”. The New York Times. Archived from the original on 23 November 2020. Retrieved 9 November 2020.
- ^ Jump up to:a b c Burger L (15 March 2020). “BioNTech in China alliance with Fosun over coronavirus vaccine candidate”. Reuters. Archived from the original on 14 November 2020. Retrieved 10 November 2020.
- ^ “Pfizer and BioNTech Celebrate Historic First Authorization in the U.S. of Vaccine to Prevent COVID-19”. Pfizer Inc. and BioNTech SE.
- ^ “Securing Singapore’s access to COVID-19 vaccines”. gov.sg. Government of Singapore. 14 December 2020. Retrieved 1 February 2021.
- ^ Jump up to:a b c d e “Deep-freeze hurdle makes Pfizer’s vaccine one for the rich”. Bloomberg. 10 November 2020. Archived from the original on 22 November 2020. Retrieved 12 November 2020.
Vaccine goes bad five days after thawing, requires two shots; Many nations face costly ramp up of cold-chain infrastructure
- ^ Jump up to:a b c “Pfizer-BioNTech COVID-19 Vaccine Vaccination Storage & Dry Ice Safety Handling”. Pfizer. Retrieved 17 December 2020.
- ^ “Information for Healthcare Professionals on Pfizer/BioNTech COVID-19 vaccine”. Government of the United Kingdom. Retrieved 29 January 2021.
- ^ “Recommendation for an Emergency Use Listing of Tozinameran (Covid-19 Mrna Vaccine (Nucleoside Modified)) Submitted by Biontech Manufacturing Gmbh” (PDF). World Health Organization. 26 January 2021.
- ^ “Australian Product Information – Comirnaty (BNT162b2 [mRNA]) COVID-19 Vaccine”(PDF). Therapeutic Goods Administration. Australian Government.
- ^ “Pfizer and BioNTech Submit COVID-19 Vaccine Stability Data at Standard Freezer Temperature to the U.S. FDA”. Pfizer (Press release). 19 February 2021. Retrieved 19 February 2021.
- ^ Gates B (30 April 2020). “The vaccine race explained: What you need to know about the COVID-19 vaccine”. The Gates Notes. Archived from the original on 14 May 2020. Retrieved 2 May 2020.
- ^ “World Health Organization timeline – COVID-19”. World Health Organization. 27 April 2020. Archived from the original on 29 April 2020. Retrieved 2 May 2020.
- ^ Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. (December 2020). “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine”. The New England Journal of Medicine. 383 (27): 2603–2615. doi:10.1056/NEJMoa2034577. PMC 7745181. PMID 33301246.
development of BNT162b2 was initiated on January 10, 2020, when the SARS-CoV-2 genetic sequence was released by the Chinese Center for Disease Control and Prevention and disseminated globally by the GISAID (Global Initiative on Sharing All Influenza Data) initiative
- ^ Bohn MK, Mancini N, Loh TP, Wang CB, Grimmler M, Gramegna M, et al. (October 2020). “IFCC Interim Guidelines on Molecular Testing of SARS-CoV-2 Infection”. Clinical Chemistry and Laboratory Medicine. 58 (12): 1993–2000. doi:10.1515/cclm-2020-1412. PMID 33027042.
- ^ “CEPI’s collaborative task force to assess COVID-19 vaccines on emerging viral strains”. BioSpectrum – Asia Edition. 23 November 2020.
the first SARS-CoV-2 viral genomes were shared via GISAID on 10 January 2020
- ^ Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S (May 2020). “The COVID-19 vaccine development landscape”. Nature Reviews. Drug Discovery. 19 (5): 305–306. doi:10.1038/d41573-020-00073-5. PMID 32273591.
- ^ Fauci AS, Lane HC, Redfield RR (March 2020). “Covid-19 – Navigating the Uncharted”. The New England Journal of Medicine. 382 (13): 1268–1269. doi:10.1056/nejme2002387. PMC 7121221. PMID 32109011.
- ^ Papadopoulos C (14 December 2020). “Chronologie – So entstand der Corona-Impfstoff von Biontech” [Chronology – That’s how the Covid-vaccine of Biontech was being developed] (in German). Südwestrundfunk. Retrieved 20 December 2020.
- ^ 《Fosun Pharma and BioNTech form COVID‑19 vaccine strategic alliance in China》(Fosun Phrama News Content , 15 March 2020) Archived 15 August 2020 at the Wayback Machine
- ^ “Germany: Investment Plan for Europe – EIB to provide BioNTech with up to €100 million in debt financing for COVID-19 vaccine development and manufacturing”. European Investment Bank. 11 June 2020. Archived from the original on 9 November 2020. Retrieved 10 November 2020.
- ^ “BioNTech gets $445 million in German funding for vaccine”. Bloomberg L.P. 15 September 2020. Archived from the original on 9 November 2020. Retrieved 10 November 2020.
- ^ “Pfizer CEO says he would’ve released vaccine data before election if possible”. Axios. 9 November 2020. Archived from the original on 10 November 2020. Retrieved 11 November 2020.
- ^ Jump up to:a b Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. (October 2020). “Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults”. Nature. 586(7830): 589–593. Bibcode:2020Natur.586..589M. doi:10.1038/s41586-020-2639-4. PMID 32785213. S2CID 221126922.
- ^ Hannah B (7 October 2020). “EMA begins rolling review of BNT162b2 COVID-19 vaccine”. European Pharmaceutical Review. Archived from the original on 11 November 2020. Retrieved 11 November 2020.
- ^ Jump up to:a b “EMA Assessment Report” (PDF). Europa (web portal). 21 December 2020. Retrieved 29 December 2020.
- ^ “Clarification of Comirnaty dosage interval”. European Medicines Agency (EMA). 28 January 2021. Retrieved 28 January 2021.
- ^ “Study to Evaluate the Safety, Tolerability, and Immunogenicity of SARS CoV-2 RNA Vaccine Candidate (BNT162b2) Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older”. ClinicalTrials.gov. Retrieved 21 February 2021.
- ^ “Pfizer and BioNTech Commence Global Clinical Trial to Evaluate COVID-19 Vaccine in Pregnant Women”. pfizer.com (Press release). 18 February 2021. Retrieved 21 February2021.
- ^ Jump up to:a b Gaebler C, Nussenzweig MC (October 2020). “All eyes on a hurdle race for a SARS-CoV-2 vaccine”. Nature. 586 (7830): 501–2. Bibcode:2020Natur.586..501G. doi:10.1038/d41586-020-02926-w. PMID 33077943. S2CID 224808629.
- ^ Jump up to:a b “China’s Fosun to end BioNTech’s COVID-19 vaccine trial, seek approval for another”. Reuters. 3 November 2020. Archived from the original on 12 December 2020. Retrieved 21 November 2020.
- ^ Jump up to:a b c World Health Organization. “Messenger RNA encoding the full-length SARS-CoV-2 spike glycoprotein” (DOC). WHO MedNet. Retrieved 16 December 2020.
- ^ Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, et al. (August 2017). “Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen”. Proceedings of the National Academy of Sciences of the United States of America. 114 (35): E7348–E7357. doi:10.1073/pnas.1707304114. PMC 5584442. PMID 28807998.
- ^ Orlandini von Niessen AG, Poleganov MA, Rechner C, Plaschke A, Kranz LM, Fesser S, et al. (April 2019). “Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3′ UTRs Identified by Cellular Library Screening”. Molecular Therapy. 27 (4): 824–836. doi:10.1016/j.ymthe.2018.12.011. PMC 6453560. PMID 30638957.
- ^ Jump up to:a b “Pfizer-BioNTech COVID-19 vaccine: Health Canada recommendations for people with serious allergies”. Health Canada. 12 December 2020.
- ^ Jump up to:a b c d Comirnaty: Product Information (PDF) (Report). European Medicines Agency(EMA). Retrieved 23 December 2020.
- ^ Public Assessment Report Authorisation for Temporary Supply COVID-19 mRNA Vaccine BNT162b2 (BNT162b2 RNA) concentrate for solution for injection (PDF). Regulation 174(Report). Medicines and Healthcare products Regulatory Agency (MHRA). 15 December 2020.
- ^ “UK medicines regulator gives approval for first UK COVID-19 vaccine”. Medicines and Healthcare products Regulatory Agency (MHRA). 2 December 2020. Archived from the original on 2 December 2020. Retrieved 2 December 2020.
- ^ Neergaard L, Kirka D (2 December 2020). “Britain OKs Pfizer vaccine and will begin shots within days”. Associated Press. Archived from the original on 6 December 2020. Retrieved 6 December 2020.
- ^ Mueller B (2 December 2020). “U.K. Approves Pfizer Coronavirus Vaccine, a First in the West”. The New York Times. Retrieved 2 December 2020.
- ^ Roberts M (2 December 2020). “Covid Pfizer vaccine approved for use next week in UK”. BBC News. Archived from the original on 2 December 2020. Retrieved 2 December 2020.
- ^ Henley J, Connolly, Jones S (3 December 2020). “European and US experts question UK’s fast-track of Covid vaccine”. The Guardian. Archived from the original on 9 December 2020. Retrieved 9 December 2020.
- ^ “First patient receives Pfizer Covid-19 vaccine”. BBC. 8 December 2020. Archivedfrom the original on 8 December 2020. Retrieved 8 December 2020.
- ^ “Vaccine vials and a virtual hug: a history of coronavirus in 15 objects”. The Guardian. 21 February 2021. Retrieved 22 February 2021.
- ^ “COVID-19 Vaccination Statistics –Week ending Sunday 20th December 2020” (PDF). NHS. 24 December 2020.
- ^ “Coronavirus en la Argentina: La ANMAT aprobo el uso de emergencia de la vacuna Pfizer”. La Nación (in Spanish). Retrieved 25 December 2020.
- ^ “TGA provisionally approves Pfizer COVID-19 vaccine”. Therapeutic Goods Administration (Press release). 25 January 2021. Retrieved 26 January 2021.
- ^ “Bahrain becomes second country to approve Pfizer COVID-19 vaccine”. Al Jazeera. Retrieved 5 December 2020.
- ^ “Drug and vaccine authorizations for COVID-19: List of applications received”. Health Canada. 9 December 2020. Retrieved 9 December 2020.
- ^ Jump up to:a b “Chile y Ecuador se adelantan en Sudamérica y autorizan la vacuna de Pfizer”. El Pais. Retrieved 17 December 2020.
- ^ “First Pfizer COVID-19 vaccines set to reach Costa Rica on Wednesday – president”. Reuters. 23 December 2020. Retrieved 24 December 2020.
- ^ “SFH authorises COVID-19 vaccine by Fosun Pharma/BioNTech for emergency use in Hong Kong”. The Government of Hong Kong (Press release). 25 January 2021. Retrieved 26 January 2021.
- ^ “Iraq grants emergency approval for Pfizer COVID-19 vaccine”. MSN. Retrieved 27 December 2020.
- ^ “Israeli Health Minister ‘pleased’ as FDA approves Pfizer COVID-19 vaccine”. The Jerusalem Post. Retrieved 28 December 2020.
- ^ “Jordan approves Pfizer-BioNTech Covid vaccine”. France 24. 15 December 2020. Retrieved 15 December 2020.
- ^ “Kuwait authorizes emergency use of Pfizer-BioNTech COVID-19 vaccine”. Arab News. 13 December 2020. Retrieved 15 December 2020.
- ^ “Mexico Approves Pfizer Vaccine for Emergency Use as Covid Surges”. Bloomberg. 12 December 2020. Retrieved 12 December 2020.
- ^ “Oman issues licence to import Pfizer BioNTech Covid vaccine – TV”. Reuters. 15 December 2020. Retrieved 16 December 2020.
- ^ “Panama approves Pfizer’s COVID-19 vaccine – health ministry”. Yahoo! Finance. Retrieved 16 December 2020.
- ^ “PH authorizes Pfizer’s COVID-19 vaccine for emergency use”. CNN Philippines. 14 January 2021.
- ^ “Qatar, Oman to receive Pfizer-BioNTech COVID-19 vaccine this week”. Reuters. Retrieved 24 December 2020.
- ^ “Saudi Arabia to Launch Its Coronavirus Vaccination Program” (in Spanish). Boomberg. Retrieved 17 December 2020.
- ^ Abdullah Z (14 December 2020). “Pfizer-BioNTech COVID-19 vaccine approved by Singapore, first shipment expected by end-December”. CNA. Retrieved 16 January 2021.
- ^ “Singapore approves use of Pfizer’s COVID-19 vaccine”. AP News. 14 December 2020. Retrieved 15 December 2020.
- ^ “Dubai approves the Pfizer-BioNTech vaccine which will be free of charge”. Emirates Woman. 23 December 2020. Retrieved 28 December 2020.
- ^ “WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access”. World Health Organization (WHO) (Press release). 31 December 2020. Retrieved 6 January 2021.
- ^ Jump up to:a b “Emergency Use Authorization for vaccines explained”. U.S. Food and Drug Administration (FDA). 20 November 2020. Archived from the original on 20 November 2020. Retrieved 20 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “Pfizer-BioNTech COVID-19 Vaccine EUA Letter of Authorization” (PDF). U.S. Food and Drug Administration (FDA). 11 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b c d “Pfizer-BioNTech COVID-19 Vaccine EUA Fact Sheet for Healthcare Providers”(PDF). Pfizer. 11 December 2020.
- ^ Sun LH, Stanley-Becker I. “CDC greenlights advisory group’s decision to recommend Pfizer vaccine for use”. The Washington Post. Retrieved 14 December 2020.
- ^ Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. (December 2020). “The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020” (PDF). MMWR. Morbidity and Mortality Weekly Report. 69 (50): 1922–24. doi:10.15585/mmwr.mm6950e2. PMC 7745957. PMID 33332292.
- ^ “COVID-19: Switzerland can start vaccinating vulnerable groups already in December”(Press release). Federal Office of Public Health. 19 December 2020. Retrieved 19 December 2020.
- ^ Erni S (23 December 2020). “90-jährige Luzernerin als erste Person in der Schweiz gegen Corona geimpft”. Neue Luzerner Zeitung. Retrieved 23 December 2020.
- ^ Pralong J (23 December 2020). “La piqûre de l’espoir pratiquée à Lucerne”. Heidi.news. Retrieved 23 December 2020.
- ^ Jump up to:a b “EMA recommends first COVID-19 vaccine for authorisation in the EU”. European Medicines Agency (EMA) (Press release). 21 December 2020. Retrieved 21 December2020.
- ^ “Comirnaty”. Union Register of medicinal products. Retrieved 8 January 2021.
- ^ “Statement by President von der Leyen on the marketing authorisation of the BioNTech-Pfizer vaccine against COVID-19”. European Commission. Retrieved 21 December 2020.
- ^ Cancian, Natália (23 February 2021). “Anvisa aprova registro da vacina da Pfizer contra Covid”. Folha de S. Paulo (in Portuguese). Retrieved 23 February 2021.
- ^ McKenna M (17 December 2020). “Vaccines Are Here. We Have to Talk About Side Effects”. Wired. Retrieved 23 December 2020.
- ^ Jump up to:a b CDC COVID-19 Response Team, Food and Drug Administration (January 2021). “Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14–23, 2020” (PDF). MMWR. Morbidity and Mortality Weekly Report. 70 (2): 46–51. doi:10.15585/mmwr.mm7002e1. PMC 7808711. PMID 33444297.
- ^ Jump up to:a b c “COVID-19 vaccine safety update: COMIRNATY” (PDF). European Medicines Agency. 28 January 2021.
- ^ Bostock N (9 December 2020). “MHRA warning after allergic reactions in NHS staff given COVID-19 vaccine”. GP. Archived from the original on 9 December 2020. Retrieved 9 December 2020.
- ^ Booth W, Cunningham E (9 December 2020). “Britain warns against Pfizer vaccine for people with history of ‘significant’ allergic reactions”. The Washington Post. Archivedfrom the original on 9 December 2020. Retrieved 9 December 2020.
- ^ Cabanillas B, Akdis C, Novak N (December 2020). “Allergic reactions to the first COVID-19 vaccine: a potential role of Polyethylene glycol?”. Allergy. doi:10.1111/all.14711. PMID 33320974. S2CID 229284320.
- ^ “First COVID-19 vaccine safety update published”. European Medicines Agency (EMA)(Press release). 28 January 2021. Retrieved 29 January 2021.
- ^ Gray B (23 November 2020). “Pfizer’s Chesterfield workforce playing a key role in coronavirus vaccine development”. St. Louis Post-Dispatch.
- ^ Jump up to:a b c d Johnson CY (17 November 2020). “A vial, a vaccine and hopes for slowing a pandemic — how a shot comes to be”. The Washington Post. Retrieved 21 December2020.
- ^ Hughes M (20 December 2020). “Andover’s piece of the vaccine: Pfizer”. The Eagle-Tribune.
- ^ Shamus KJ (13 December 2020). “Historic journey: Pfizer prepares to deliver 6.4 million doses of COVID-19 vaccines”. Detroit Free Press.
- ^ Mullin R (25 November 2020). “Pfizer, Moderna ready vaccine manufacturing networks”. Chemical & Engineering News. Washington, D.C.: American Chemical Society. Retrieved 21 December 2020.
- ^ Jump up to:a b c Weise, Elizabeth (7 February 2021). “Pfizer expects to cut COVID-19 vaccine production time by close to 50% as production ramps up, efficiencies increase”. USA Today.
- ^ “BioNTech to Acquire GMP Manufacturing Site to Expand COVID-19 Vaccine Production Capacity in First Half 2021 | BioNTech”. investors.biontech.de. Retrieved 5 February2021.
- ^ “Statement on Manufacturing | BioNTech”. investors.biontech.de. Retrieved 5 February2021.
- ^ Erman M, Ankur B (22 July 2020). “U.S. to pay Pfizer, BioNTech $1.95 bln for millions of COVID-19 vaccine doses”. Reuters. Archived from the original on 22 July 2020. Retrieved 22 July 2020.
- ^ “U.S. Government Engages Pfizer to Produce Millions of Doses of COVID-19 Vaccine”. US Department of Health and Human Services. 22 July 2020. Archived from the original on 22 July 2020. Retrieved 23 July 2020.
- ^ Nazaryan A (9 November 2020). “So is Pfizer part of Operation Warp Speed or not? Yes and no”. Yahoo!. Archived from the original on 10 November 2020. Retrieved 9 November 2020.
- ^ Pleitgen F (11 November 2020). “EU agrees to buy 300 million doses of the Pfizer/BioNTech Covid-19 vaccine”. CNN. Archived from the original on 24 November 2020. Retrieved 26 November 2020.
- ^ “Japan and Pfizer reach COVID-19 vaccine deal to treat 60 million people”. The Japan Times. 1 August 2020. Archived from the original on 10 November 2020. Retrieved 21 November 2020.
- ^ Tasker JP (9 November 2020). “Trudeau says promising new Pfizer vaccine could be ‘light at the end of the tunnel‘“. CBC News. Archived from the original on 9 November 2020. Retrieved 9 November 2020.
- ^ “Pfizer and BioNTech to Supply Singapore with their BNT162b2 mRNA-based Vaccine Candidate to Combat COVID-19”. pfizer.com.sg. Pfizer Singapore. 14 December 2020. Retrieved 1 February 2021.
- ^ de Salud S. “233. Firma secretario de Salud convenio con Pfizer para fabricación y suministro de vacuna COVID-19”. gob.mx (in Spanish). Retrieved 17 December 2020.
- ^ Ng E (27 August 2020). “Fosun Pharma to supply Covid-19 vaccine to Hong Kong, Macau once approved”. South China Morning Post. Archived from the original on 20 November 2020. Retrieved 21 November 2020.
- ^ Ting V, Lau C, Wong O (11 December 2020). “Hong Kong buys 15 million Covid-19 vaccine doses from Sinovac, Pfizer”. South China Morning Post. Retrieved 18 December2020.
- ^ “BioNTech and Fosun Pharma to Supply China with mRNA-based COVID-19 Vaccine”(Press release). BioNTech. 16 December 2020. Retrieved 16 December 2020.
- ^ “Pfizer-BioNTech COVID-19 Vaccine Frequently Asked Questions”. U.S. Food and Drug Administration. 11 December 2020. Retrieved 29 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b “Extra dose from vials of Comirnaty COVID-19 vaccine”. European Medicines Agency (EMA). 8 January 2021. Retrieved 8 January 2021.
- ^ “AIFA, possibile ottenere almeno 6 dosi da ogni flaconcino del vaccino BioNTech/Pfizer”. aifa.gov.it (in Italian). Retrieved 29 December 2020.
- ^ “Global information about Comirnaty”. Comirnaty IE. 8 January 2021. Retrieved 16 January 2021.
- ^ “Comirnaty Package Insert” (PDF). BioNTech Manufacturing GmbH.
- ^ Rowland C (22 January 2021). “Biden wants to squeeze an extra shot of vaccine out of every Pfizer vial. It won’t be easy”. The Washington Post. Retrieved 29 January 2021.
- ^ Kollewe J. “Pfizer and BioNTech’s vaccine poses global logistics challenge”. The Guardian. Archived from the original on 10 November 2020. Retrieved 10 November2020.
- ^ Newey S (8 September 2020). “Daunting task of distribution exposed as it emerges some vaccines must be ‘deep frozen’ at −70C”. The Telegraph. Archived from the original on 9 November 2020. Retrieved 10 November 2020.
- ^ “How China’s COVID-19 could fill the gaps left by Pfizer, Moderna, AstraZeneca”. Fortune. 5 December 2020. Archived from the original on 12 December 2020. Retrieved 5 December 2020.
- ^ “Pfizer’s Vaccine Is Out of the Question as Indonesia Lacks Refrigerators: State Pharma Boss”. Jakarta Globe. 22 November 2020. Archived from the original on 7 December 2020. Retrieved 5 December 2020.
- ^ “Pfizer Has Offered South Africa Discounted Covid-19 Vaccines”. Bloomberg. 4 January 2021. Retrieved 5 January 2021.
- ^ Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. (December 2020). “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine”. N Engl J Med. 383 (27): 2603–2615. doi:10.1056/NEJMoa2034577. PMC 7745181. PMID 33301246.
- ^ World Health Organization (2020). “International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 124 – COVID-19 (special edition)”(PDF). WHO Drug Information. 34 (3): 666. Archived (PDF) from the original on 27 November 2020. Retrieved 23 November 2020.
- ^ “Pfizer and BioNTech Receive Authorization in the European Union for COVID-19 Vaccine” (Press release). BioNTech. 21 December 2020. Retrieved 26 December 2020 – via GlobeNewswire.
- ^ Bulik BS (23 December 2020). “The inside story behind Pfizer and BioNTech’s new vaccine brand name, Comirnaty”. FiercePharma. Retrieved 25 December 2020.
- ^ “Comirnaty COVID-19 mRNA Vaccine”. Comirnaty Global. Retrieved 31 December2020.
External links
“Tozinameran”. Drug Information Portal. U.S. National Library of Medicine.
- Global Information About Pfizer–BioNTech COVID‑19 Vaccine (also known as BNT162b2) Pfizer
- Comirnaty assessment report European Medicines Agency Committee for Medicinal Products for Human Use
- A Phase 1/2/3 Study to Evaluate the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID‑19 in Healthy Individuals Pfizer clinical protocol
- Pfizer Vaccince News, updates and tracking of Israel’s vaccinaion campaign
- “How the Pfizer-BioNTech Covid-19 Vaccine Works”. The New York Times.

A vial of the Pfizer–BioNTech COVID‑19 vaccine
|
|
| Vaccine description | |
|---|---|
| Target disease | COVID‑19 |
| Type | mRNA |
| Clinical data | |
| Trade names | Comirnaty[1][2] |
| Other names | BNT162b2, COVID-19 mRNA vaccine (nucleoside-modified) |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Intramuscular |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem SID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Part of a series on the |
| COVID-19 pandemic |
|---|
|
/////////Tozinameran, APPROVALS 2021, JAPAN 2021, Comirnaty, Coronavirus disease, COVID-19, BNT162b2 , BNT162b2 SARS-CoV-2 Vaccine, RNA ingredient BNT-162B2
The Pfizer-BioNTech COVID-19 vaccine (Tozinameran, INN), also known as BNT162b2, is one of four advanced mRNA-based vaccines developed through “Project Lightspeed,” a joint program between Pfizer and BioNTech.2,3 Tozinameran is a nucleoside modified mRNA (modRNA) vaccine encoding an optimized full-length version of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein. It is designed to induce immunity against SARS-CoV-2, the virus responsible for causing COVID-19.2 The modRNA is formulated in lipid nanoparticles for administration via intramuscular injection in two doses, three weeks apart.1,3
Tozinameran is undergoing evaluation in clinical trials in both the USA (NCT04368728) and Germany (NCT04380701).4,5 Tozinameran received fast track designation by the U.S. FDA on July 13, 2020.6 On December 11, 2020, the FDA issued an Emergency Use Authorization (EUA) based on 95% efficacy in clinical trials and a similar safety profile to other viral vaccines over a span of approximately 2 months.1 Tozinameran was granted an EUA in the UK on December 2, 2020,8 and in Canada on December 9, 20207 for active immunization against SARS-CoV-2.12
Currently, sufficient data are not available to determine the longevity of protection against COVID-19, nor direct evidence that the vaccine prevents the transmission of the SARS-CoV-2 virus from one individual to another.9 Fact sheets for caregivers, recipients, and healthcare providers are now available.10,11
Tozinameran has not yet been fully approved by any country. In both the UK and Canada, Tozinameran is indicated under an interim authorization for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals aged 16 years and older.7,8
On December 11, 2020, the U.S. Food and Drug Administration granted emergency use authorization (EUA) for Tozinameran to prevent COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients aged 16 years and above.9 Safety and immune response information for adolescents 12-15 years of age will follow, and studies to further explore the administration of Tozinameran in pregnant women, children under 12 years of age, and those in special risk groups will be evaluated in the future.1
This vaccine should only be administered where appropriate medical treatment for immediate allergic reactions are immediately available in the case of an acute anaphylactic reaction after vaccine administration.12 Tozinameran administration should be postponed in any individual suffering from an acute febrile illness. Its use should be carefully considered in immunocompromised individuals and individuals with a bleeding disorder or on anticoagulant therapy. Appropriate medical treatment should be readily available in case of an anaphylactic reaction following vaccine administration.7,8
Tozinameran contains nucleoside modified mRNA (modRNA) encapsulated in lipid nanoparticles that deliver the modRNA into host cells. The lipid nanoparticle formulation facilitates the delivery of the RNA into human cells.12 Once inside these cells, the modRNA is translated by host machinery to produce the SARS-CoV-2 spike (S) protein antigen, which is subsequently recognized by the host immune system. Tozinameran has been shown to elicit both neutralizing antibody and cellular immune responses to the S protein, which helps protect against subsequent SARS-CoV-2 infection.7,8
Tozinameran is a nucleoside modified mRNA (modRNA) vaccine encoding an optimized full-length version of the SARS-CoV-2 spike (S) protein, translated and expressed in cells in vaccinated individuals to produce the S protein antigen against which an immune response is mounted. As with all vaccines, protection cannot be guaranteed in all recipients, and full protection may not occur until at least seven days following the second dose.7,8
In U.S. clinical trials, the vaccine was 95% effective in preventing COVID-19; eight COVID-19 cases occurred in the vaccine group and 162 cases occurred in the placebo group. Of the total 170 COVID-19 cases, one case in the vaccine group and three cases in the placebo group were considered to be severe infections.1,9
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 10. doi: 10.1056/NEJMoa2034577. [PubMed:33301246]
- Gen Eng News: BNT162 vaccine candidates [Link]
- BioNTech BNT162 Update [Link]
- Clinical Trial NCT04368728 [Link]
- Clinical Trial NCT04380701 [Link]
- FDA fast track designation: BNT162b1 and BNT162b2 [Link]
- Health Canada Interim Product Monograph: BNT162b2 SARS-CoV-2 Vaccine [Link]
- MHRA Interim Product Monograph: BNT162b2 SARS-CoV-2 Vaccine [Link]
- FDA News Release: FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine [Link]
- Pfizer: Fact Sheet for Healthcare Providers Administering Vaccine, Pfizer-BioNtech COVID-19 vaccine [Link]
- Pfizer: Fact Sheet for Recipients and Caregivers, Pfizer BioNTech COVID-19 vaccine [Link]
- FDA Emergency Use Authorization: Full EUA Prescribing information, Pfizer-BioNTech COVID-19 vaccine [Link]
-
PHASE STATUS PURPOSE CONDITIONS COUNT 2 Active Not Recruiting Prevention Coronavirus Disease 2019 (COVID‑19) 1 2, 3 Active Not Recruiting Prevention Coronavirus Disease 2019 (COVID‑19) 1 1, 2 Active Not Recruiting Prevention Coronavirus Disease 2019 (COVID‑19) 1 1, 2 Recruiting Treatment Coronavirus Disease 2019 (COVID‑19) / Protection Against COVID-19 and Infections With SARS CoV 2 / Respiratory Tract Infections (RTI) / RNA Virus Infections / Vaccine Adverse Reaction / Viral Infections / Virus Diseases 1