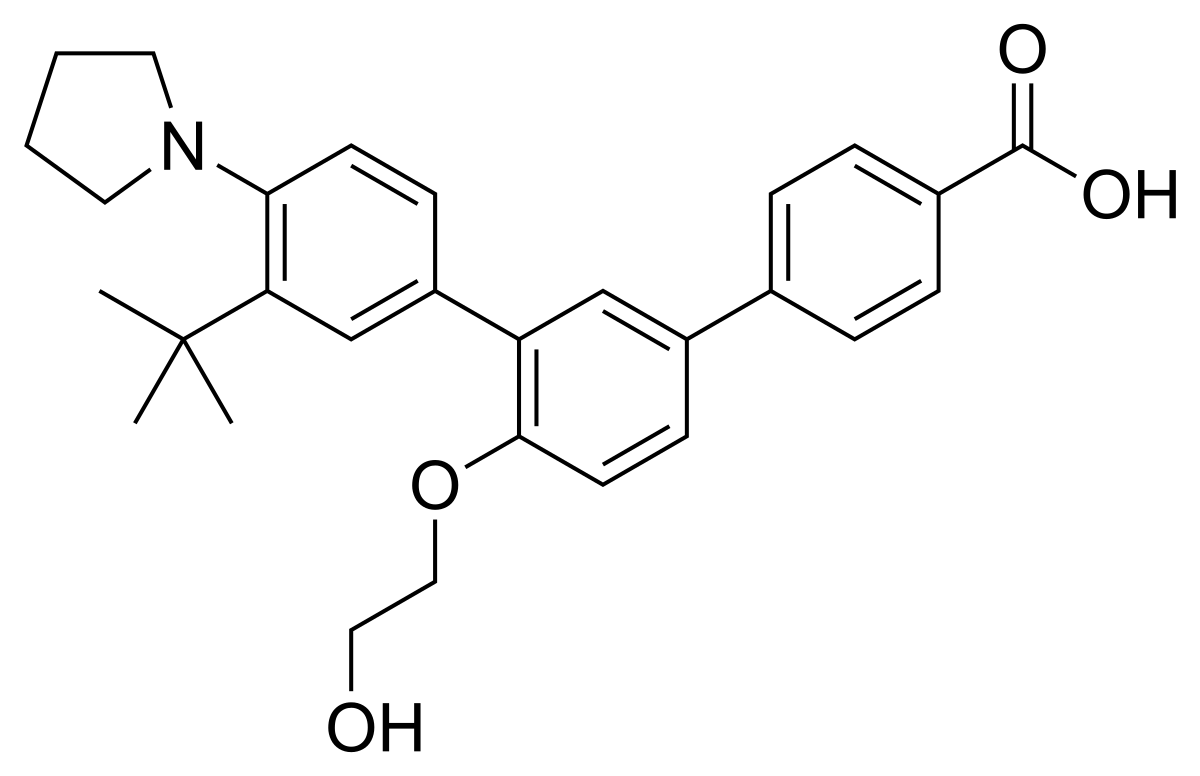
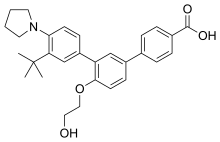
Trifarotene
CAS 895542-09-3
3”-Tert-butyl-4′-(2-hydroxyethoxy)-4”-(pyrrolidin-1-yl)(1,1′:3′,1”)terphenyl-4-carboxylic acid
3′-[3-tert-butyl-4-(pyrrolidin-1-yl)phenyl]-4′-(2-hydroxyethoxy)-[1,1′-biphenyl]-4-carboxylic acid
UNII-0J8RN2W0HK,
Galderma Research & Development
459.5766
C29 H33 N O4
- CD-5789
- CD5789
Trifarotene, sold under the brand name Aklief, is a medication for the topical treatment of acne vulgaris in those nine years of age and older.[1] It is a retinoid;[2] more specifically, it is a fourth generation selective retinoic acid receptor (RAR)-γ agonist.[3]
It was approved for medical use in the United States in 2019,[1][4][5] but is not approved in the European Union as of January 2021.[6] Trifarotene was granted orphan drug designation for the treatment of congenital ichthyosis by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).[7][8]
State Solid
Experimental Properties
| PROPERTY | VALUE | SOURCE |
|---|---|---|
| melting point (°C) | 245C | FDA Label |
| pKa | 5.69 (pKa1) | FDA Label |
USFDA
The drug substance, trifarotene, a terphenyl acid derivative, is a retinoic
acid receptor (RAR) aQonist and is classified as a rotenoid. Trifarotene
intended as a drug for the treatment of acne vulgaris. Since trifarotene
has not been previously approved as an active ingredient in any drug
product in the United States, it is classified as a new molecular entity
(NME).
Trifarotene is produced as a white to off-white to slightly yellow crystalline
powder. It is slightly soluble in acetone, ethanol, and toluene, very slight
soluble in isopropanol, and practically insoluble in water (tiJT4
1
Cb><“JTrifarotene is nonhygroscopic and has pKa1 of 5.69 and pKa2 of 4.55. The chemical name
for trifarotene is 4-{3-[3-tert-butyl-4-(pyrrolidin-1-yl) phenyl]-4-(2-
hydroxyethoxy) phenyl} benzoic acid. It has the chemical formula of
C29H33NQ4, the molecular weiQht of 459.59, …………https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211527Orig1s000ChemR.pdf
Prescription Products
For treatment of congenital ichthyosis, PRECLINICAL, Galderma Res & Dev,
Galderma announced that the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation status for the company’s trifarotene molecule for the treatment of congenital ichthyosis. Based on this decision, Galderma plans to implement a clinical development plan, reinforcing its commitment to exploring new treatment options for rare diseases, as well as meeting the needs of all patients with skin diseases over the course of their lives.
Galderma治療先天性魚鱗癬的Trifarotene分子取得FDA的孤兒藥資格認定
http://news.msn.com.tw/market3773054.aspx
The company’s molecule trifarotene is a selective agonist of the gamma retinoic acid receptor (RAR-gamma), which is currently in clinical development for use in other more common dermatological conditions. It is the drug’s retinoid functionality and potent keratolytic properties that make it a potentially viable treatment of the lamellar ichthyosis pathology. Galderma has already initiated the program for investigating the treatment of lamellar ichthyosis with trifarotene and is currently working in collaboration with regulatory authorities to implement an innovative and expedient clinical development plan.
Ichthyoses comprise a large group of skin scaling disorders with diverse etiologies. The stereotypic pathophysiology is epidermal hyperplasia and abnormal desquamation, leading to visible accumulation of squames (scales) on the skin’s surface. Congenital ichthyosis is a term used to refer to a specific group of rare inherited forms of ichthyoses that are generally more severe than non-inherited forms of the disease. Lamellar ichthyosis is one such disorder that falls within the congenital ichthyosis category. Lamellar ichthyosis is recognized as a severe disease which persists throughout life. After birth, during the first post-natal weeks, the hyperkeratotic (colloidion) membrane patients are typically born with, is gradually shed and is replaced by scaling and lichenification that involves the entire body, including face, scalp, palms and soles. While usually not life threatening, lamellar ichthyosis can result in disability, partial deafness, poor adaptation to environmental conditions (due to hypohydrosis), severe discomfort (pruritus, fissuring of the skin), and significant psycho-social impact. The estimated prevalence of LI in the US is in the range of 1 per 100,000 to 1 per 200,000 persons.
Synthesis Reference
Thoreau, E. et. al. Structure-based design of Trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorganic & Medicinal Chemistry Letters, Volume 28, Issue 10. 2018. Pages 1736-1741
https://www.sciencedirect.com/science/article/abs/pii/S0960894X18303482
Trifarotene – Synthetic Route 1

Synthetic Description
Reference: Biadatti, Thibaud; Dumais, Laurence; Soulet, Catherine; Talano, Sandrine; Daver, Sebastien. Preparation of [1,1′:3′,1”]terphenyl-4-carboxylic acid and esters a novel ligands modulating retinoic acid receptors (RAR), and use thereof in human medicine and in cosmetics. Assignee Galderma Research & Development, S.N.C., Fr. WO 2006066978. (2016).
PATENT
WO 2006066978
http://www.google.com/patents/WO2006066978A1?cl=en
Example 25 – 3″-ter.-Butyl-4′-(2-hvdroxyethoxy)-4″-pyrrolidin-1-ylM,1′:3′,1″1- terphenyl-4-carboxylic acid
In a manner similar to that of Example 6b, by reacting 500 mg (0.9 mmol) of ethyl 4′-(2- acetoxyethoxy)-3″-terf-butyl-4″-pyrrolidin-1 -yl[1 , 1 ‘;3’, 1 “]terphenyl-4-carboxylate with
300 mg (8 mmol) of sodium hydroxide, 242 mg of 3″-tert-butyl-4′-(2-hydroxyethoxy)-4″- pyrrolidin-1-yl[1l1′;3′,1″]terphenyl-4-carboxylic acid are obtained (yield = 55 %) in the form of a white solid (m.p. = 2230C).
1H NMR (DMSO. 400 MHz): 1.43 (s, 9H); 1.90 (m, 4H); 3.0 (m, 4H); 3.73 (d, J=4.7Hz, 2H); 4.1 (m, 2H); 4.7 (s, 1H); 7.2 (d, 1H, J=8.6Hz); 7.48 (m, 2H); 7.59 (d, J=1.6Hz, 1H); 7.64 (d, J=UHz, 1H); 7.68 (dd, J=2Hz, 7.8Hz, 1H); 7.82 (d, J=8.3Hz, 2H); 7.99 (d, J=8.4Hz, 2H).
PATENT
WO 2013178759
http://www.google.com/patents/WO2013178759A1?cl=en
PATENT
WO 2013178758
http://www.google.com/patents/WO2013178758A1?cl=en
PATENT
WO 2013178760
http://www.google.com/patents/WO2013178760A1?cl=en
The details of skin application are given in the table below.
SYN
New Drug Approvals for 2019: Synthesis and Clinical Applications
New Drug Approvals for 2019: Synthesis and Clinical Applications
Shuo Yuan, Bin Yu, Hong-Min Liu
PII: S0223-5234(20)30639-5
DOI: https://doi.org/10.1016/j.ejmech.2020.112667
Reference: EJMECH 112667
To appear in: European Journal of Medicinal Chemistry
Trifarotene (Aklief). In October 2019, trifarotene, a topical retinoid that
selectively targets retinoic acid receptor gamma (RAR-γ), was approved by the FDA
for the treatment of acne vulgaris [142]. The drug was developed and marketed by
Galderma Pharmaceutical in Switzerland. Trifarotene is considered as the first of the
‘fourth-generation’ retinoids due to its uniquely selective agonism at RAR-γ. The
selective agonism leads to downstream alterations, confering improved efficacy and
reduced side effects [143]. In two phase 3 clinical trials of 2420 patients with
moderate acne on the face and trunk, trifarotene was well tolerated and significantly
reduced inflammatory lesions as early as two weeks on the face and four weeks on the
back, shoulders and chest compared to vehicle (p<0.05) [144].
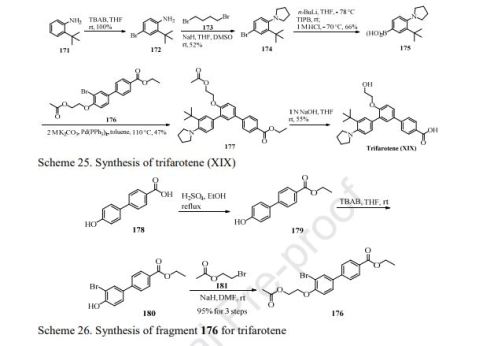
The synthetic approach of this drug was disclosed by Galderma Research &
Development (Scheme 25) [145]. Bromination of commercially available
2-(tert-butyl)aniline 171 gave 4-bromo-2-(tert-butyl)aniline 172 in quantitative yield,
which then reacted with 1-dibromobutane 173 to give phenylpyrrolidine 174 in 52%
yield. Miyaura reaction of 174 was realized by employing n-BuLi and triisopropyl
borate (TIPB) followed by washed with aqueous HCl, resulting in arylboronic acid
adduct 175 in 66% yield. Treatment of 175 with aromatic bromide 176 in the presence
of Pd(PPh3)4 gave the coupling product 177 in 47% yield, which then underwent
hydrolysis delivering trifarotene (XIX) in 55% yield.
The preparation of coupling partner 176 is depicted in Scheme 26. Esterification of
4-hydroxy-4-biphenylcarboxylic acid 178 gave ethyl benzoate derivative 179 upon
treatment with catalytic H2SO4 in the refluxing EtOH [145]. The resulting ester was
subjected to treatment with tetrabutylammonium bromide (TBAB) in THF, resulting
in bromide 180 in good yields, further NaH-mediated Williamson ether synthesis with
2-bromoethyl acetate 181 gave 176 in 95% yield.
[142] L.J. Scott, Trifarotene: first approval, Drugs 79 (2019) 1905-1909.
[143] E. Thoreau, J.M. Arlabosse, C. Bouix-Peter, S. Chambon, L. Chantalat, S.
Daver, L. Dumais, G. Duvert, A. Feret, G. Ouvry, J. Pascau, C. Raffin, N.
Rodeville, C. Soulet, S. Tabet, S. Talano, T. Portal, Structure-based design of
trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of
acne, Bioorg. Med. Chem. Lett. 28 (2018) 1736-1741.
[144] J. Tan, D. Thiboutot, G. Popp, M. Gooderham, C. Lynde, J.D. Rosso, J. Weiss,
U. Blume-Peytavi, J. Weglovska, S. Johnson, L. Parish, D. Witkowska, N.S.
Colon, A.A. Saenz, F. Ahmad, M. Graeber, L.S. Gold, Randomized phase 3
evaluation of trifarotene 50 µg/g cream treatment of moderate facial and truncal
acne, J. Am. Acad. Dermatol. 80 (2019) 1691-1699.
[145] T. Biadatti, L. Dumais, C. Soulet, S. Talano, S. Daver, Novel ligands that
modulate rar receptors, and use thereof in human medicine and in cosmetics,
2006. WO2006066978.
| WO2006066978A1 * | Dec 21, 2005 | Jun 29, 2006 | Galderma Res & Dev | Novel ligands that modulate rar receptors, and use thereof in human medicine and in cosmetics |
| EP0826366A2 | Aug 1, 1997 | Mar 4, 1998 | Unilever N.V. | Cosmetic compositions containing hydroxy acid or retinoid |
| EP0989846A2 | Sep 22, 1998 | Apr 5, 2000 | E-L Management Corp. | Non-irritating cosmetic and pharmaceutical compositions |
| EP1831149A1 | Dec 21, 2005 | Sep 12, 2007 | Galderma Research & Development | Novel ligands that modulate rar receptors and use thereof in human medicine and in cosmetics |
| FR2915682A1 * | Title not available | |||
| US5851538 | Dec 29, 1995 | Dec 22, 1998 | Advanced Polymer Systems, Inc. | Retinoid formulations in porous microspheres for reduced irritation and enhanced stability |
| WO1999010308A1 * | Aug 21, 1998 | Mar 4, 1999 | Bernardon Jean Michel | Biphenyl derivatives substituted by an aromatic or heteroaromatic radical and pharmaceutical and cosmetic compositions containing same |
| US6150413 * | May 26, 1998 | Nov 21, 2000 | Centre International De Recherches Dermatologiques | Treatment of dermatological, rheumatic, respiratory, cardiovascular, bone and ophthalmological disorders, as well as mammalian skin and hair conditions; 4-(4-(biphenyl-2-yl)but-3-en-1-ynyl)benzoic acid, for example |
 |
|
| CLINICAL DATA | |
|---|---|
| TRADE NAMES | Aklief |
| OTHER NAMES | CD5789 |
| AHFS/DRUGS.COM | Monograph |
| MEDLINEPLUS | a620004 |
| LICENSE DATA |
|
| PREGNANCY CATEGORY |
|
| ROUTES OF ADMINISTRATION |
Topical |
| DRUG CLASS | Skin and mucous membrane agents |
| ATC CODE | |
| LEGAL STATUS | |
| LEGAL STATUS | |
| IDENTIFIERS | |
| CAS NUMBER | |
| PUBCHEM CID | |
| DRUGBANK | |
| CHEMSPIDER | |
| UNII | |
| KEGG | |
| CHEMBL | |
| COMPTOX DASHBOARD (EPA) | |
| ECHA INFOCARD | 100.278.901 |
| CHEMICAL AND PHYSICAL DATA | |
| FORMULA | C29H33NO4 |
| MOLAR MASS | 459.586 g·mol−1 |
| 3D MODEL (JSMOL) | |
References
- ^ Jump up to:a b “Drug Trials Snapshots: Aklief”. U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Trifarotene Monograph
- ^ Scott LJ (November 2019). “Trifarotene: First Approval”. Drugs. 79 (17): 1905–1909. doi:10.1007/s40265-019-01218-6. PMID 31713811.
- ^ “Aklief (trifarotene) FDA Approval History”. Drugs.com. 7 October 2019. Retrieved 19 November 2019.
- ^ “Drug Approval Package: Aklief”. U.S. Food and Drug Administration (FDA). 21 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
- ^ “Trifarotene”. European Medicines Agency. Retrieved 17 June 2020.
- ^ “Trifarotene Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 19 August 2020.
- ^ “EU/3/20/2264”. European Medicines Agency (EMA). 12 August 2020. Retrieved 19 August 2020.
External links
- “Trifarotene”. Drug Information Portal. U.S. National Library of Medicine (NLM).
- Aubert J, Piwnica D, Bertino B, Blanchet-Rethore S, Carlavan I, Deret S, Dreno B, Gamboa B, Jomard A, Luzy AP, Mauvais P, Mounier C, Pascau J, Pelisson I, Portal T, Rivier M, Rossio P, Thoreau E, Vial E, Voegel JJ: Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-gamma agonist trifarotene. Br J Dermatol. 2018 Aug;179(2):442-456. doi: 10.1111/bjd.16719. Epub 2018 Jul 4. [PubMed:29974453]
- Balak DMW: Topical trifarotene: a new retinoid. Br J Dermatol. 2018 Aug;179(2):231-232. doi: 10.1111/bjd.16733. [PubMed:30141539]
- Blume-Peytavi U, Fowler J, Kemeny L, Draelos Z, Cook-Bolden F, Dirschka T, Eichenfield L, Graeber M, Ahmad F, Alio Saenz A, Rich P, Tanghetti E: Long-term safety and efficacy of trifarotene 50 mug/g cream, a first-in-class RAR-gamma selective topical retinoid, in patients with moderate facial and truncal acne. J Eur Acad Dermatol Venereol. 2019 Jul 15. doi: 10.1111/jdv.15794. [PubMed:31306527]
- Tan J, Thiboutot D, Popp G, Gooderham M, Lynde C, Del Rosso J, Weiss J, Blume-Peytavi U, Weglovska J, Johnson S, Parish L, Witkowska D, Sanchez Colon N, Alio Saenz A, Ahmad F, Graeber M, Stein Gold L: Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019 Jun;80(6):1691-1699. doi: 10.1016/j.jaad.2019.02.044. Epub 2019 Feb 22. [PubMed:30802558]
- Chien A: Retinoids in Acne Management: Review of Current Understanding, Future Considerations, and Focus on Topical Treatments J Drugs Dermatol. 2018 Dec 1;17(12):s51-55. [PubMed:30586483]
- FDA Approved Drugs: Aklief® [Link]
| FORM | ROUTE | STRENGTH |
|---|---|---|
| Cream | Topical | 50 mcg |
| Cream | Topical | 50 ug/1g |
| Cream | Topical | 50 MICROGRAMMI/G |
| PHASE | STATUS | PURPOSE | CONDITIONS | COUNT |
|---|---|---|---|---|
| 4 | Enrolling by Invitation | Treatment | Acne Vulgaris | 1 |
| 3 | Completed | Treatment | Acne Vulgaris | 4 |
| 2 | Completed | Treatment | Acne Vulgaris | 1 |
| 2 | Recruiting | Treatment | Lamellar Ichthyosis | 1 |
| 1 | Completed | Treatment | Malignant Lymphomas | 1 |
















