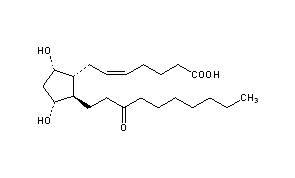
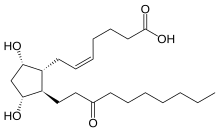
Unoprostone
- Molecular FormulaC22H38O5
- Average mass382.534 Da
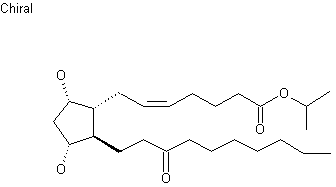
Unoprostone (INN) is a prostaglandin analogue. Its isopropyl ester, unoprostone isopropyl, was marketed under the trade name Rescula for the management of open-angle glaucoma and ocular hypertension, but is now discontinued in the US.[1]
Unoprostone isopropyl is a prostaglandin analogue. Ophthalmic Solution 0.15% is a synthetic docosanoid. Unoprostone isopropyl has the chemical name isopropyl (+)-(Z)-7-[(1R,2R,3R,5S)-3,5 dihydroxy-2-(3-oxodecyl)cyclopentyl]-5-heptenoate. The main indication of Unoprostane is treatment of glucoma.
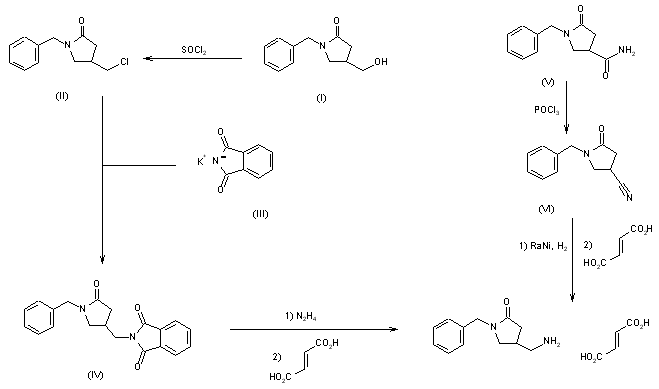
This compound can be prepared by two different ways: 1) The reaction of 1-benzyl-4-(hydroxymethyl)pyrrolidin-2-one (I) with SOCl2 in refluxing dichloromethane gives 1-benzyl-4-(chloromethyl)pyrrolidin-2-one (II), which is condensed with potassium phthalimide (III) in DMF yielding 1-benzyl-4-(phthalimidomethyl)pyrrolidin-2-one (IV). Finally, this compound is treated with hydrazine in ethanol and neutralized with fumaric acid. 2) The dehydration of 1-benzyl-2-oxo-pyrrolidine-4-carboxamide (V) with POCl3 in hot DMF gives 1-benzyl-4-cyanopyrrolidine-2-one (VI), which is reduced with H2 and RaNi in methanol – NH3 and neutralized with fumaric acid. EP 0289349; JP 1989151552; US 5001153; US 5106869
syn 2
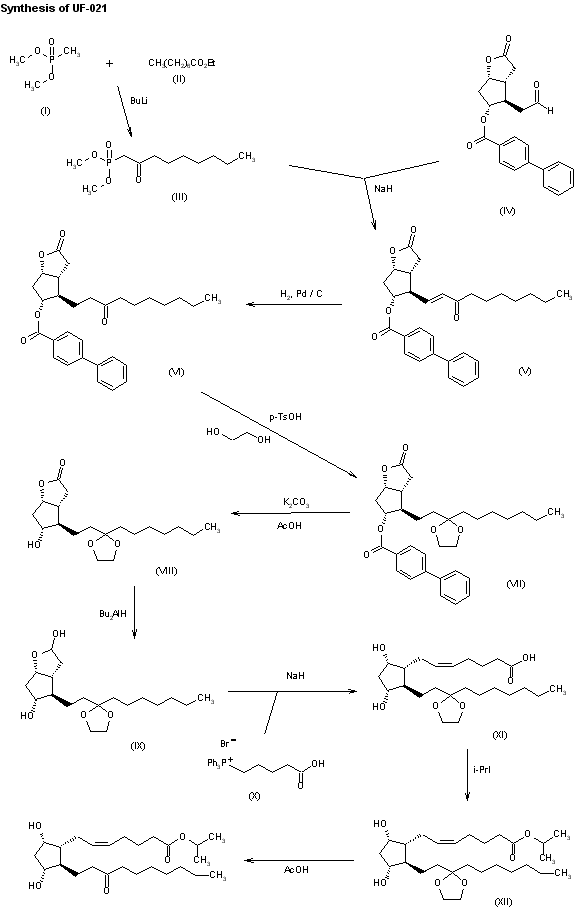
The condensation of dimethyl methylphosphonate (I) with ethyl octanoate (II) by means of butyllithium in THF gives dimethyl 2-oxononylphosphonate (III), which is condensed with the protected aldehyde (IV) by means of NaH in THF, yielding the unsaturated ketone (V). The hydrogenation of (V) with H2 over Pd/C in ethyl acetate affords the corresponding saturated ketone (VI), which is treated with ethylene glycol and p-toluenesulfonic acid to give the cyclic ketal (VII). The mild hydrolysis of (VII) with K2CO3 and acetic acid gives the alcohol derivative (VIII); the reduction of the lactone group of (VIII) with dibutylaluminum hydride in toluene affords the lactol (IX), which is condensed with (4-carboxybutyl)triphenylphosphonium bromide (X) by means of NaH in DMSO yielding the protected prostaglandin (XI). Esterification of (XI) with isopropyl iodide and DBU in acetonitrile gives the precursor (XII), which is finally deprotected with acetic acid in THF – water.
References
 |
|
| Clinical data | |
|---|---|
| Trade names | Rescula |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration |
Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 14 min |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.227.145 |
| Chemical and physical data | |
| Formula | C22H38O5 |
| Molar mass | 382.534 g/mol g·mol−1 |
| 3D model (JSmol) | |
///////////////Antiglaucoma, ocular hypertension, UF-021, Unoprostone
CCCCCCCC(=O)CC[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O
CCCCCCCC(=O)














