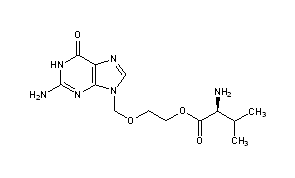
VALACYCLOVIR
Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster(shingles).[1] It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases.[1] It is taken by mouth.[1]
Common side effects include headache and vomiting.[1] Severe side effects may include kidney problems.[1] Use in pregnancy appears to be safe.[1] It is a prodrug, which works after being converted to aciclovir in a person’s body.[1]
Valaciclovir was patented in 1987 and came into medical use in 1995.[2][3] It is available as a generic medication.[4] A month supply in the United Kingdom costs the NHS about £3 as of 2019.[4] In the United States the wholesale cost of this amount is about US$2.80.[5]In 2016 it was the 168th most prescribed medication in the United States with more than 3 million prescriptions.[6]
Medical uses
Valaciclovir is used for the treatment of HSV and VZV infections, including:[7]
- Oral and genital herpes simplex (treatment and prevention)
- Reduction of HSV transmission from people with recurrent infection to uninfected individuals
- Herpes zoster (shingles): the typical dosage for treatment of herpes is 1,000 mg orally three times a day for seven consecutive days.[8]
- Prevention of cytomegalovirus following organ transplantation
- Prevention of herpesviruses in immunocompromised people (such as those undergoing cancer chemotherapy)[9]
- Chickenpox in children ages 2-18)[10]
It has shown promise as a treatment for infectious mononucleosis[11][12][13] and is preventively administered in suspected cases of herpes B virus exposure.[14]
Valaciclovir is not recommended in Bell’s palsy due to lack of benefit.[15]
Adverse effects
Common adverse drug reactions (≥1% of people) associated with valaciclovir are the same as for aciclovir, its active metabolite. They include: nausea, vomiting, diarrhea and headache. Infrequent adverse effects (0.1–1% of patients) include: agitation, vertigo, confusion, dizziness, edema, arthralgia, sore throat, constipation, abdominal pain, rash, weakness and/or renal impairment. Rare adverse effects (<0.1% of patients) include: coma, seizures, neutropenia, leukopenia, tremor, ataxia, encephalopathy, psychotic symptoms, crystalluria, anorexia, fatigue, hepatitis, Stevens–Johnson syndrome, toxic epidermal necrolysis and/or anaphylaxis.[7]
Pharmacology
Valaciclovir belongs to a family of molecules. Valaciclovir is a prodrug, an esterified version of aciclovir that has greater oral bioavailability (about 55%) than aciclovir.[10] It is converted by esterases to the active drug, aciclovir, and the amino acid, valine, via hepatic first-pass metabolism. Aciclovir is selectively converted into a monophosphate form by viral thymidine kinase, which is more effective (3000 times) in phosphorylation of aciclovir than cellular thymidine kinase. Subsequently, the monophosphate form is further phosphorylated into a disphosphate by cellular guanylate kinase and then into the active triphosphate form, aciclo-GTP, by cellular kinases.[10]
Mechanism of action
Aciclo-GTP, the active triphosphate metabolite of aciclovir, is a very potent inhibitor of viral DNA replication. Aciclo-GTP competitively inhibits and inactivates the viral DNA polymerase.[10] Its monophosphate form also incorporates into the viral DNA, resulting in chain termination. It has also been shown that the viral enzymes cannot remove aciclo-GMP from the chain, which results in inhibition of further activity of DNA polymerase. Aciclo-GTP is fairly rapidly metabolized within the cell, possibly by cellular phosphatases.[16]
Aciclovir is active against most species in the herpesvirus family. In descending order of activity:[17]
- Herpes simplex virus type I (HSV-1)
- Herpes simplex virus type II (HSV-2)
- Varicella zoster virus (VZV)
- Epstein–Barr virus (EBV)
- Cytomegalovirus (CMV)
The drug is predominantly active against HSV and, to a lesser extent, VZV. It is only of limited efficacy against EBV and CMV. However, valacyclovir has recently been shown to lower or eliminate the presence of the Epstein–Barr virus in subjects afflicted with acute mononucleosis, leading to a significant decrease in the severity of symptoms.[11][12][13] Although it can prevent the establishment of viral latency, acyclovir therapy has not proven effective at eradicating latent viruses in nerve ganglia.[17]
As of 2005, resistance to valaciclovir has not been significant. Mechanisms of resistance in HSV include deficient viral thymidine kinase and mutations to viral thymidine kinase and/or DNA polymerase that alter substrate sensitivity.[18]
It also is used for herpes B virus postexposure prophylaxis.[14]
History
Valaciclovir was patented in 1987 and came into medical use in 1995.[2][3] It is available as a generic medication.[4] A month supply in the United Kingdom costs the NHS about £3 as of 2019.[4] In the United States the wholesale cost of this amount is about US$2.80.[5] In 2019, it was the 168th most prescribed medication in the United States with more than 3 million prescriptions.[6]
Formulations
It is marketed by GlaxoSmithKline under the trade names Valtrex and Zelitrex. Valaciclovir has been available as a generic drug in the U.S. since November 25, 2009.[19]
Valtrex is offered in 500 mg and 1 gram tablets, with the active ingredient valacyclovir hydrochloride. The inactive ingredients include carnauba wax, colloidal silicon dioxide, crospovidone, FD&C Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide.[20]
SYN
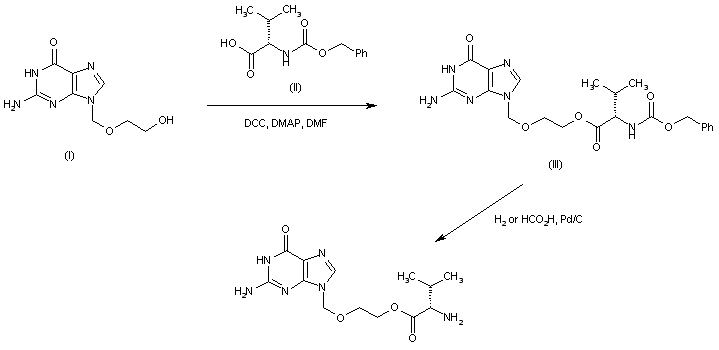
Acyclovir (I) was coupled with N-Cbz-L-valine (II) in the presence of DCC and DMAP to afford the Cbz-protected valyl ester (III). The N-benzyloxycarbonyl group of (III) was then removed by either hydrogenation over Pd/C or by transfer hydrogenation in the presence of formic acid. AU 8820978; EP 0308065; EP 0596542; JP 1989068373; JP 1991115284; US 4957924; US 5061708
SYN 2
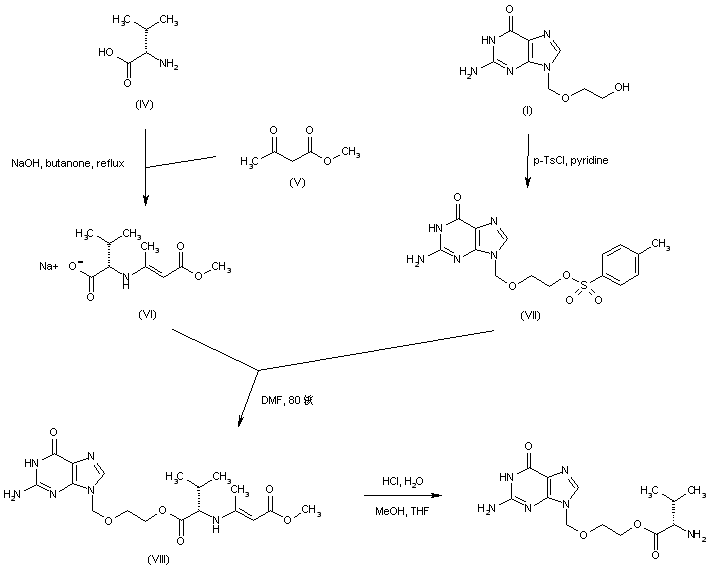
In an alternative procedure, condensation of L-valine (IV) with methyl acetoacetate (V) in the presence of NaOH produced the enamine-protected valine sodium salt (VI). Condensation of (VI) with the tosylate (VII), (prepared from acyclovir (I) and tosyl chloride) afforded ester (VIII). Then, acidic hydrolysis of the enaminoester moiety of (VIII) furnished the target valine ester. Similar procedures were also reported using omega-mesyl and omega-chloro acyclovir.
SYN3
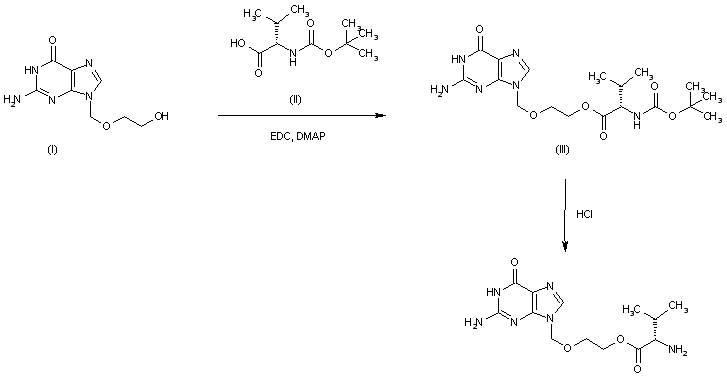
The esterification of acyclovir (I) with N-(tert-butoxycarbonyl)-L-valine (II) by means of EDC, TEA and DMAP in DMF gives the corresponding ester (III) which is finally deprotected by means of HCl in water to afford the target valacyclovir.
Valaciclovir
- Synonyms:Valacyclovir, BW-256U, 256 U 87
- ATC:J05AB11
- MW:324.34 g/mol
- CAS-RN:124832-26-4
- InChI Key:HDOVUKNUBWVHOX-QMMMGPOBSA-N
- InChI:InChI=1S/C13H20N6O4/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20)/t8-/m0/s1
Derivatives
monohydrochloride
- Formula:C13H20N6O4 • HCl
- MW:360.80 g/mol
- CAS-RN:124832-27-5
Substance Classes
Synthesis Path
References
-
- US 4 957 924 (Burroughs Wellcome; 18.9.1990; GB-prior. 15.8.1987).
- EP 308 065 (Wellcome Found. Ltd; appl. 12.8.1988; GB-prior. 15.8.1987, 5.11.1987).
-
combination with lamotrigine:
- WO 9 505 179 (Wellcome Found. Ltd; appl. 17.8.1994; GB-prior. 18.8.1993).
-
water-dispersible tablets:
- WO 9 213 527 (Wellcome Found. Ltd; appl. 29.1.1992; GB-prior. 30.1.1991, 22.11.1991, 25.11.1991).
-
medical use for preventing post herpetic neuralgia:
- GB 2 282 759 (SmithKline Beecham; appl. 14.10.1994; GB-prior. 16.10.1993).
References
- ^ Jump up to:a b c d e f g “Valacyclovir Hydrochloride Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Retrieved 17 March 2019.
- ^ Jump up to:a b Long, Sarah S.; Pickering, Larry K.; Prober, Charles G. (2012). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 1502. ISBN 1437727026.
- ^ Jump up to:a b Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495.
- ^ Jump up to:a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 625–626. ISBN 9780857113382.
- ^ Jump up to:a b “NADAC as of 2019-02-27”. Centers for Medicare and Medicaid Services. Retrieved 3 March 2019. Cite error: The named reference “NADAC2019” was defined multiple times with different content (see the help page).
- ^ Jump up to:a b “The Top 300 of 2019”. clincalc.com. Retrieved 22 December 2018. Cite error: The named reference “:1” was defined multiple times with different content (see the help page).
- ^ Jump up to:a b Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3[page needed]
- ^ Lille, H. Martina; Wassilew, Sawko W. (2006). “Antiviral therapies of shingles in dermatology”. In Gross, Gerd; Doerr, H.W. (eds.). Herpes zoroster: recent aspects of diagnosis and control. Monographs in virology. 26. Basel (Switzerland): Karger Publishers. p. 124. ISBN 978-3-8055-7982-7. Retrieved January 1, 2012.
- ^ Elad S, Zadik Y, Hewson I, et al. (August 2010). “A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea”. Support Care Cancer. 18 (8): 993–1006. doi:10.1007/s00520-010-0900-3. PMID 20544224.
- ^ Jump up to:a b c d “VALTREX (valacyclovir hydrochloride) Caplets -GSKSource”. gsksource.com. Retrieved 2019-08-02.
- ^ Jump up to:a b Balfour et al. (December 2005) A controlled trial of valacyclovir in infectious mononucleosis. Presented at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC., December 18, 2005. Abstract V1392
- ^ Jump up to:a b Simon, Michael W.; Robert G. Deeter; Britt Shahan (March 2003). “The Effect of Valacyclovir and Prednisolone in Reducing Symptoms of EBV Illness In Children: A Double-Blind, Placebo-Controlled Study” (PDF). International Pediatrics. 18 (3): 164–169.[dead link]
- ^ Jump up to:a b Balfour HH, Hokanson KM, Schacherer RM, et al. (May 2007). “A virologic pilot study of valacyclovir in infectious mononucleosis”. Journal of Clinical Virology. 39 (1): 16–21. doi:10.1016/j.jcv.2007.02.002. PMID 17369082.
- ^ Jump up to:a b “B Virus—First Aid and Treatment—Herpes B—CDC”. Retrieved June 6, 2015.
- ^ Baugh, Reginald F.; Basura, Gregory J.; Ishii, Lisa E.; Schwartz, Seth R.; Drumheller, Caitlin Murray; Burkholder, Rebecca; Deckard, Nathan A.; Dawson, Cindy; Driscoll, Colin (November 2013). “Clinical Practice Guideline: Bell’s Palsy”. Otolaryngology–Head and Neck Surgery. 149 (3_suppl): S1–S27. doi:10.1177/0194599813505967. ISSN 0194-5998.
In summary, antiviral therapy alone (acyclovir or valacyclovir) is not recommended in the treatment of Bell’s palsy due to lack of effectiveness of currently available drugs, unnecessary cost, and the potential for drug-related complications.
- ^ http://www.uscnk.us/protein-antibody-elisa/Valaciclovir-%28VCV%29-V511.htm[permanent dead link]
- ^ Jump up to:a b O’Brien JJ, Campoli-Richards DM (March 1989). “Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy”. Drugs. 37 (3): 233–309. doi:10.2165/00003495-198937030-00002. PMID 2653790.
- ^ Sweetman, Sean C., ed. (2005). Martindale: the complete drug reference (34th ed.). London: Pharmaceutical Press. ISBN 0-85369-550-4. OCLC 56903116.[page needed]
- ^ Ahmed, Rumman (November 27, 2009). “Ranbaxy Launches Generic Valtrex in U.S.”The Wall Street Journal. Retrieved January 16, 2010.
- ^ “Valtrex Prescribing Information” (PDF). GlaxoSmithKline. September 2008. Retrieved May 7, 2009.
External links
 |
|
| Clinical data | |
|---|---|
| Trade names | Valtrex, Zelitrex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 55% |
| Protein binding | 13–18% |
| Metabolism | Liver (to aciclovir) |
| Elimination half-life | <30 minutes (valaciclovir); 2.5–3.6 hours (aciclovir) |
| Excretion | Kidney 40–50% (aciclovir), faecal 47% (aciclovir) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.114.479 |
| Chemical and physical data | |
| Formula | C13H20N6O4 |
| Molar mass | 324.336 g/mol g·mol−1 |
| 3D model (JSmol) | |
//////////////Valacyclovir HCl, hydrochloride salt of L-valyl ester, aciclovir, GlaxoSmithKline ,















