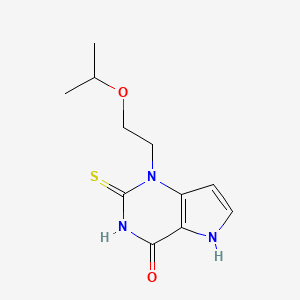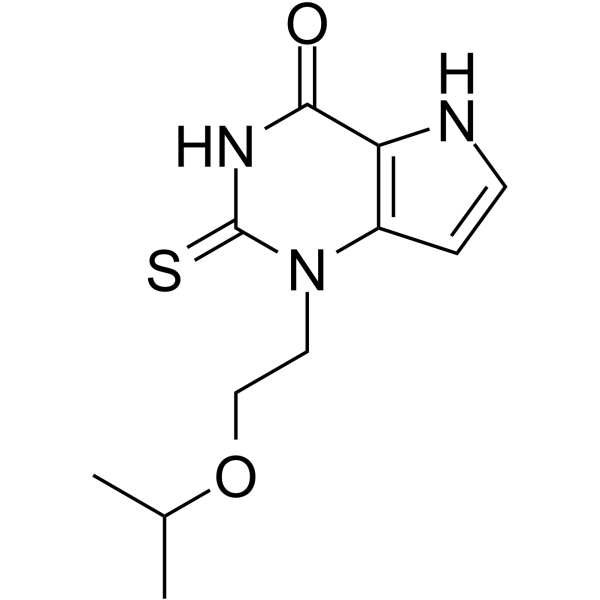
Verdiperstat
AZD 3241; BHV-3241
CAS No. : 890655-80-8
1-(2-propan-2-yloxyethyl)-2-sulfanylidene-5H-pyrrolo[3,2-d]pyrimidin-4-one
4H-Pyrrolo[3,2-d]pyrimidin-4-one, 1,2,3,5-tetrahydro-1-[2-(1-methylethoxy)ethyl]-2-thioxo-
1-(2-isopropoxyethyl)-2-thioxo-1,2,3,5-tetrahydro-pyrrolo[3,2-d] pyrimidin-4-one
l-(2-Isopropoxyethyl)-2-thioxo-l,2,3,5-tetrahydro-pyrrolo[3,2-d]pyrimidin-4-one
- Molecular FormulaC11H15N3O2S
- Average mass253.321 Da
AZD-3241, BHV-3421, UNII-TT3345YXVR, TT3345YXVR, BHV-3241, WHO 10251
- OriginatorAstraZeneca
- DeveloperAstraZeneca; Biohaven Pharmaceuticals
- ClassAntiparkinsonians; Ethers; Organic sulfur compounds; Pyrimidinones; Small molecules
- Mechanism of ActionPeroxidase inhibitors
- Orphan Drug StatusYes – Multiple system atrophy
- Phase IIIMultiple system atrophy
- Phase II/IIIAmyotrophic lateral sclerosis
- DiscontinuedParkinson’s disease
- 23 Jun 20213574186: Added patent info and HE
- 23 Jun 2021Biohaven Pharmaceuticals has patents pending for the composition of matter of verdiperstat, pharmaceutical compositions and various neurological diseases in Europe, Japan and other countries
- 01 Nov 2020Brigham and Women’s Hospital plans a phase I trial for Multiple System Atrophy in USA , (NCT04616456)
Key facts
| Active substance |
1-(2-isopropoxyethyl)-2-thioxo-1,2,3,5-tetrahydro-pyrrolo[3,2-d] pyrimidin-4-one (verdiperstat)
|
| Intented use |
Treatment of multiple system atrophy
|
| Orphan designation status |
Positive
|
| EU designation number |
EU/3/14/1404
|
| Date of designation |
16/12/2014
|
| Sponsor |
Biohaven Pharmaceutical Ireland DAC |
VERDIPERSTAT
For Initial Indications in Multiple System Atrophy (MSA) and Amyotrophic Lateral Sclerosis (ALS)
Verdiperstat is a first-in-class, potent, selective, brain-penetrant, irreversible myeloperoxidase (MPO) enzyme inhibitor. Verdiperstat was progressed through Phase 2 clinical trials by AstraZeneca. Seven clinical studies were completed by AstraZeneca, including four Phase 1 studies in healthy subjects, two Phase 2a studies in subjects with Parkinson’s Disease, and one Phase 2b study in subjects with MSA. These Phase 2 clinical studies provide evidence that verdiperstat achieves peripheral target engagement (i.e., reduces MPO specific activity in plasma) and central target engagement in the brain and offer proof of its mechanism of action (i.e., reduce microglial activation and neuroinflamation).
A Phase 3 clinical trial to evaluate the efficacy of verdiperstat in MSA is currently ongoing. A Phase 2/3 trial to evaluate the efficacy of verdiperstat in ALS is currently ongoing as part of the HEALEY ALS Platform Trial.
Verdiperstat has received Fast Track and Orphan Drug designations by the U.S. Food and Drug Administration (FDA) and the European Medicine Agency due to the unmet medical needs in MSA.
Verdiperstat Overview
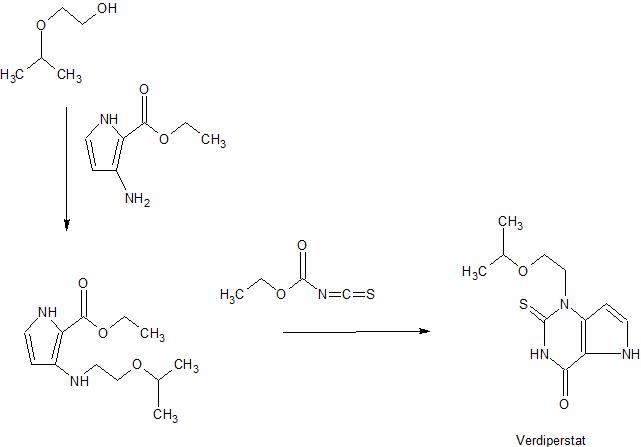
/////////verdiperstat, вердиперстат , فيرديبيرستات , 维地泊司他 , WHO 10251, AZD-3241, BHV-3421, UNII-TT3345YXVR, TT3345YXVR, BHV-3241, AZD 3241, BHV 3241, BHV 3421
CC(C)OCCN1C2=C(C(=O)NC1=S)NC=C2