|
PGQEHPNARK YKGANKKGLS KGCFGLKLDR IGSMSGLGC
(Disulfide bridge: 23-39) |

H-Pro-Gly-Gln-Glu-His-Pro-Asn-Ala-Arg-Lys-Tyr-Lys-Gly-Ala-Asn-Lys-Lys-Gly-Leu-Ser-Lys-Gly-Cys(1)-Phe-Gly-Leu-Lys-Leu-Asp-Arg-Ile-Gly-Ser-Met-Ser-Gly-Leu-Gly-Cys(1)-OH
PGQEHPNARKYKGANKKGLSKGCFGLKLDRIGSMSGLGC
H-PGQEHPNARKYKGANKKGLSKGC(1)FGLKLDRIGSMSGLGC(1)-OH
PEPTIDE1{P.G.Q.E.H.P.N.A.R.K.Y.K.G.A.N.K.K.G.L.S.K.G.C.F.G.L.K.L.D.R.I.G.S.M.S.G.L.G.C}$PEPTIDE1,PEPTIDE1,23:R3-39:R3$$$
L-prolyl-glycyl-L-glutaminyl-L-alpha-glutamyl-L-histidyl-L-prolyl-L-asparagyl-L-alanyl-L-arginyl-L-lysyl-L-tyrosyl-L-lysyl-glycyl-L-alanyl-L-asparagyl-L-lysyl-L-lysyl-glycyl-L-leucyl-L-seryl-L-lysyl-glycyl-L-cysteinyl-L-phenylalanyl-glycyl-L-leucyl-L-lysyl-L-leucyl-L-alpha-aspartyl-L-arginyl-L-isoleucyl-glycyl-L-seryl-L-methionyl-L-seryl-glycyl-L-leucyl-glycyl-L-cysteine (23->39)-disulfide
(4R,10S,16S,19S,22S,28S,31S,34S,37S,40S,43S,49S,52R)-52-[[2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-4-amino-2-[[(2S)-2-[[2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-5-amino-5-oxo-2-[[2-[[(2S)-pyrrolidine-2-carbonyl]amino]acetyl]amino]pentanoyl]amino]-4-carboxybutanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carbonyl]amino]-4-oxobutanoyl]amino]propanoyl]amino]-5-carbamimidamidopentanoyl]amino]hexanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]hexanoyl]amino]acetyl]amino]propanoyl]amino]-4-oxobutanoyl]amino]hexanoyl]amino]hexanoyl]amino]acetyl]amino]-4-methylpentanoyl]amino]-3-hydroxypropanoyl]amino]hexanoyl]amino]acetyl]amino]-40-(4-aminobutyl)-49-benzyl-28-[(2S)-butan-2-yl]-31-(3-carbamimidamidopropyl)-34-(carboxymethyl)-16,22-bis(hydroxymethyl)-10,37,43-tris(2-methylpropyl)-19-(2-methylsulfanylethyl)-6,9,12,15,18,21,24,27,30,33,36,39,42,45,48,51-hexadecaoxo-1,2-dithia-5,8,11,14,17,20,23,26,29,32,35,38,41,44,47,50-hexadecazacyclotripentacontane-4-carboxylic acid
Vosoritide
| Formula |
C176H290N56O51S3
|
|---|---|
| CAS |
1480724-61-5
|
| Mol weight |
4102.7254
|
Voxzogo, 2021/8/26 EU APPROVED
| Product details | |
|---|---|
| Name |
Voxzogo
|
| Agency product number |
EMEA/H/C/005475
|
| Active substance |
Vosoritide
|
| International non-proprietary name (INN) or common name |
vosoritide
|
| Therapeutic area (MeSH) |
Achondroplasia
|
| Anatomical therapeutic chemical (ATC) code |
M05BX
|
| Orphan |
This medicine was designated an orphan medicine. This means that it was developed for use against a rare, life-threatening or chronically debilitating condition or, for economic reasons, it would be unlikely to have been developed without incentives. For more information, see Orphan designation. |
| Publication details | |
|---|---|
| Marketing-authorisation holder |
BioMarin International Limited
|
| Date of issue of marketing authorisation valid throughout the European Union |
26/08/2021
|
On 24 January 2013, orphan designation (EU/3/12/1094) was granted by the European Commission to BioMarin Europe Ltd, United Kingdom, for modified recombinant human C-type natriuretic peptide for the treatment of achondroplasia.
The sponsorship was transferred to BioMarin International Limited, Ireland, in February 2019.
This medicine is now known as Vosoritide.
The medicinal product has been authorised in the EU as Voxzogo since 26 August 2021.
PEPTIDE
|
Treatment of Achondroplasia
modified recombinant human C-type natriuretic peptide (CNP) |
Vosoritide, sold under the brand name Voxzogo, is a medication used for the treatment of achondroplasia.[1]
The most common side effects include injection site reactions (such as swelling, redness, itching or pain), vomiting and decreased blood pressure.[1]
Vosoritide was approved for medical use in the European Union in August 2021.[1][2]
Voxzogo is a medicine for treating achondroplasia in patients aged 2 years and older whose bones are still growing.
Achondroplasia is an inherited disease caused by a mutation (change) in a gene called fibroblast growth-factor receptor 3 (FGFR3). The mutation affects growth of almost all bones in the body including the skull, spine, arms and legs resulting in very short stature with a characteristic appearance.
Achondroplasia is rare, and Voxzogo was designated an ‘orphan medicine’ (a medicine used in rare diseases) on 24 January 2013. Further information on the orphan designation can be found here: ema.europa.eu/medicines/human/orphan-designations/EU3121094.
Voxzogo contains the active substance vosoritide.

Medical uses
Vosoritide is indicated for the treatment of achondroplasia in people two years of age and older whose epiphyses are not closed.[1]
Mechanism of action
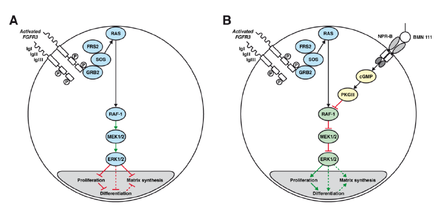
A: Chondrocyte with constitutionally active FGFR3 that down-regulates its development via the MAPK/ERK pathway
B: Vosoritide (BMN 111) blocks this mechanism by binding to the atrial natriuretic peptide receptor B (NPR-B), which subsequently inhibits the MAPK/ERK pathway at the RAF-1 protein.[3]
Vosoritide works by binding to a receptor (target) called natriuretic peptide receptor type B (NPR-B), which reduces the activity of fibroblast growth factor receptor 3 (FGFR3).[1] FGFR3 is a receptor that normally down-regulates cartilage and bone growth when activated by one of the proteins known as acidic and basic fibroblast growth factor. It does so by inhibiting the development (cell proliferation and differentiation) of chondrocytes, the cells that produce and maintain the cartilaginous matrix which is also necessary for bone growth. Children with achondroplasia have one of several possible FGFR3 mutations resulting in constitutive (permanent) activity of this receptor, resulting in overall reduced chondrocyte activity and thus bone growth.[3]
The protein C-type natriuretic peptide (CNP), naturally found in humans, reduces the effects of over-active FGFR3. Vosoritide is a CNP analogue with the same effect but prolonged half-life,[3] allowing for once-daily administration.[4]
Chemistry
Vosoritide is an analogue of CNP. It is a peptide consisting of the amino acids proline and glycine plus the 37 C-terminal amino acids from natural human CNP. The complete peptide sequence is
with a disulfide bridge between positions 23 and 39 (underlined).[5] The drug must be administered by injection as it would be rendered ineffective by the digestive system if taken by mouth.
History
Vosoritide is being developed by BioMarin Pharmaceutical and, being the only available causal treatment for this condition, has orphan drug status in the US as well as the European Union.[1][2][6] As of September 2015, it is in Phase II clinical trials.[7][4]
Society and culture
Controversy
Some people with achondroplasia, as well as parents of children with this condition, have reacted to vosoritide’s study results by saying that dwarfism is not a disease and consequently does not need treatment.[8]
Research
Vosoritide has resulted in increased growth in a clinical trial with 26 children. The ten children receiving the highest dose grew 6.1 centimetres (2.4 in) in six months, compared to 4.0 centimetres (1.6 in) in the six months before the treatment (p=0.01).[9] The body proportions, more specifically the ratio of leg length to upper body length – which is lower in achondroplasia patients than in the average population – was not improved by vosoritide, but not worsened either.[7][10]
As of September 2015, it is not known whether the effect of the drug will last long enough to result in normal body heights,[10] or whether it will reduce the occurrence of achondroplasia associated problems such as ear infections, sleep apnea or hydrocephalus. This, together with the safety of higher doses, is to be determined in further studies.[4]
References
- ^ Jump up to:a b c d e f g “Voxzogo EPAR”. European Medicines Agency. 23 June 2021. Retrieved 9 September 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Jump up to:a b “European Commission Approves BioMarin’s Voxzogo (vosoritide) for the Treatment of Children with Achondroplasia from Age 2 Until Growth Plates Close”. BioMarin Pharmaceutical Inc. (Press release). 27 August 2021. Retrieved 9 September 2021.
- ^ Jump up to:a b c Lorget F, Kaci N, Peng J, Benoist-Lasselin C, Mugniery E, Oppeneer T, et al. (December 2012). “Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia”. American Journal of Human Genetics. 91 (6): 1108–14. doi:10.1016/j.ajhg.2012.10.014. PMC 3516592. PMID 23200862.
- ^ Jump up to:a b c Clinical trial number NCT02055157 for “A Phase 2 Study of BMN 111 to Evaluate Safety, Tolerability, and Efficacy in Children With Achondroplasia (ACH)” at ClinicalTrials.gov
- ^ “International Nonproprietary Names for Pharmaceutical Substances (INN): List 112” (PDF). WHO Drug Information. 28 (4): 539. 2014.
- ^ “Food and Drug Administration Accepts BioMarin’s New Drug Application for Vosoritide to Treat Children with Achondroplasia” (Press release). BioMarin Pharmaceutical. 2 November 2020. Retrieved 9 September 2021 – via PR Newswire.
- ^ Jump up to:a b Spreitzer H (6 July 2015). “Neue Wirkstoffe – Vosoritid”. Österreichische Apothekerzeitung (in German) (14/2015): 28.
- ^ Pollack A (17 June 2015). “Drug Accelerated Growth in Children With Dwarfism, Pharmaceutical Firm Says”. The New York Times.
- ^ “BMN 111 (vosoritide) Improves Growth Velocity in Children With Achondroplasia in Phase 2 Study”. BioMarin. 17 June 2015.
- ^ Jump up to:a b “Vosoritid” (in German). Arznei-News.de. 20 June 2015.
External links
- “Vosoritide”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Trade names | Voxzogo |
| Other names | BMN-111 |
| Routes of administration |
Subcutaneous injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C176H290N56O51S3 |
| Molar mass | 4102.78 g·mol−1 |
| 3D model (JSmol) | |
/////////Vosoritide, Voxzogo, PEPTIDE, ボソリチド (遺伝子組換え) , восоритид , فوسوريتيد , 伏索利肽 , APPROVALS 2021, EU 2021, BMN 111, ORPHAN DRUG
CCC(C)C1C(=O)NCC(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NCC(=O)NC(CSSCC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCNC(=N)N)CC(=O)O)CC(C)C)CCCCN)CC(C)C)CC2=CC=CC=C2)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(CO)NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(CCCCN)NC(=O)C(CC(=O)N)NC(=O)C(C)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(CC3=CC=C(C=C3)O)NC(=O)C(CCCCN)NC(=O)C(CCCNC(=N)N)NC(=O)C(C)NC(=O)C(CC(=O)N)NC(=O)C4CCCN4C(=O)C(CC5=CN=CN5)NC(=O)C(CCC(=O)O)NC(=O)C(CCC(=O)N)NC(=O)CNC(=O)C6CCCN6)C(=O)O)CC(C)C)CO)CCSC)CO