
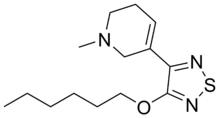
Xanomeline (LY-246,708; Lumeron, Memcor)
CAS 131986-45-3
- Molecular FormulaC14H23N3OS
- Average mass281.417 Da
Xanomeline oxalate
CAS No.:141064-23-5,
Molecular Weight, :371.45,
Molecular Formula, :C16H25N3O5S
5‐[4‐(hexyloxy)‐1,2,5‐thiadiazol‐3‐yl]‐1‐methyl‐1,2,3,6‐tetrahydropyridine; oxalic acid
SEE………..
Title: Xanomeline
CAS Registry Number: 131986-45-3
CAS Name: 3-[4-(Hexyloxy)-1,2,5-thiadiazol-3-yl]-1,2,5,6-tetrahydro-1-methylpyridine
Molecular Formula: C14H23N3OS
Molecular Weight: 281.42
Percent Composition: C 59.75%, H 8.24%, N 14.93%, O 5.69%, S 11.39%
Literature References: Selective muscarinic M1-receptor agonist.
Prepn: P. Sauerberg, P. H. Olesen, EP384288 (1990 to Ferrosan); eidem,US5043345 (1991 to Novo Nordisk); eidemet al.,J. Med. Chem.35, 2274 (1992).
Prepn of crystalline tartrate: L. M. Osborne et al.,WO9429303 (1994 to Novo Nordisk).
Muscarinic receptor binding study: H. E. Shannon et al.,J. Pharmacol. Exp. Ther.269, 271 (1994). Pharmacology: F. P. Bymaster et al.,ibid. 282.
HPLC determn in plasma: C. L. Hamilton et al.,J. Chromatogr.613, 365 (1993).
Derivative Type: Oxalate
CAS Registry Number: 141064-23-5
Molecular Formula: C14H23N3OS.C2H2O4
Molecular Weight: 371.45
Percent Composition: C 51.74%, H 6.78%, N 11.31%, O 21.54%, S 8.63%
Properties: Crystals from acetone, mp 148°.
Melting point: mp 148°
Derivative Type: (+)-L-Hydrogen tartrate
CAS Registry Number: 152854-19-8
Additional Names: Xanomeline tartrate
Manufacturers’ Codes: LY-246708; NNC-11-0232
Trademarks: Lomeron (Lilly); Memcor (Lilly)
Molecular Formula: C14H23N3OS.C4H6O6
Molecular Weight: 431.50
Percent Composition: C 50.10%, H 6.77%, N 9.74%, O 25.95%, S 7.43%
Properties: Crystals from 2-propanol, mp 95.5°.
Melting point: mp 95.5°
Therap-Cat: Cholinergic; nootropic.
Keywords: Cholinergic; Nootropic.
SYNTHESIS WILL BE UPDATED

| EP 0384288; US 5260311; US 5264444; US 5328925, US 5834495; WO 9429303, EP 0687265; JP 1996507298; WO 9420495 | |
 |
|
| The reaction of pyridine-3-carbaldehyde (I) with KCN in acetic acid, followed by a treatment with NH4Cl in aqueous NH4OH yields 2-amino-2-(3-pyridyl)acetonitrile (II), which is cyclized to 3-chloro-4-(3-pyridyl)-1,2,5-thiadiazole (III) by a treatment with S2Cl2 in DMF. The reaction of (III) with sodium hexyloxide in hexanol yields 3-(hexyloxy)-4-(3-pyridyl)-1,2,5-thiadiazole (IV), which is treated with methyl iodide in acetone to afford the corresponding N-methylpyridinium salt (V). Finally, this compound is hydrogenated with NaBH4 in ethanol and salified with oxalic or L-tartaric acid in acetone or isopropanol. | |

PAPER
http://www.mdpi.com/1420-3049/6/3/142/htm
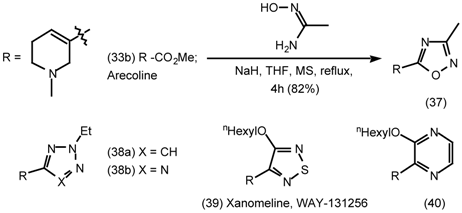
Xanomeline (39) has emerged as one of the most potent unbridged arecoline derivatives. It has higher potency and efficacy for m1 and m4 than for m2, m3 and m5 receptor subtypes [73], binds to the m1receptor subtype uniquely tightly [74,75] and stimulates phosphoinositide hydrolysis in the brain. In cells containing human m1 receptors which are stably expressing amyloid precursor protein (APP), xanomeline (39) stimulates APP release with a potency 1000 greater than carbachol and reduces the secretion of Aβ by 46% [76] (cf 2.6 Central nervous system). In patients with Alzheimer’s disease, it halted cognitive decline and reduced behavioural symptoms such as hallucinations, delusions and vocal outbursts [77,78]. As might be expected there have been numerous attempts to prepare analogues with comparable potency and efficacy. Transplanting the thiadiazole ring of xanomeline to a range of bicyclic amines reduced selectivity [79,80] as did the use of pyrazine analogues (40) [81].
Paper
J Med Chem 1992,35(12),2274-83
see http://pubs.acs.org/doi/pdf/10.1021/jm00090a019
PAPER
Classics in Chemical Neuroscience: Xanomeline
Abstract

Xanomeline (1) is an orthosteric muscarinic acetylcholine receptor (mAChR) agonist, often referred to as M1/M4-preferring, that received widespread attention for its clinical efficacy in schizophrenia and Alzheimer’s disease (AD) patients. Despite the compound’s promising initial clinical results, dose-limiting side effects limited further clinical development. While xanomeline, and related orthosteric muscarinic agonists, have yet to receive approval from the FDA for the treatment of these CNS disorders, interest in the compound’s unique M1/M4-preferring mechanism of action is ongoing in the field of chemical neuroscience. Specifically, the promising cognitive and behavioral effects of xanomeline in both schizophrenia and AD have spurred a renewed interest in the development of safer muscarinic ligands with improved subtype selectivity for either M1 or M4. This Review will address xanomeline’s overall importance in the field of neuroscience, with a specific focus on its chemical structure and synthesis, pharmacology, drug metabolism and pharmacokinetics (DMPK), and adverse effects.
PAPER
References
- Jump up^ Farde L, Suhara T, Halldin C, et al. (1996). “PET study of the M1-agonists [11C]xanomeline and [11C]butylthio-TZTP in monkey and man”. Dementia (Basel, Switzerland). 7 (4): 187–95. PMID 8835881.
- Jump up^ Jakubík J, Michal P, Machová E, Dolezal V (2008). “Importance and prospects for design of selective muscarinic agonists” (PDF). Physiological Research / Academia Scientiarum Bohemoslovaca. 57 Suppl 3: S39–47. PMID 18481916.
- Jump up^ Woolley ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA (January 2009). “Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice”. European Journal of Pharmacology. 603 (1-3): 147–9. PMID 19111716. doi:10.1016/j.ejphar.2008.12.020.
- Jump up^ Heinrich JN, Butera JA, Carrick T, et al. (March 2009). “Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists”. European Journal of Pharmacology. 605 (1-3): 53–6. PMID 19168056. doi:10.1016/j.ejphar.2008.12.044.
- Jump up^ Grant MK, El-Fakahany EE (October 2005). “Persistent binding and functional antagonism by xanomeline at the muscarinic M5 receptor”. The Journal of Pharmacology and Experimental Therapeutics. 315 (1): 313–9. PMID 16002459. doi:10.1124/jpet.105.090134.
- Jump up^ Lieberman JA, Javitch JA, Moore H (August 2008). “Cholinergic agonists as novel treatments for schizophrenia: the promise of rational drug development for psychiatry”. The American Journal of Psychiatry. 165 (8): 931–6. PMID 18676593. doi:10.1176/appi.ajp.2008.08050769.
- Jump up^ Messer WS (2002). “The utility of muscarinic agonists in the treatment of Alzheimer’s disease”. Journal of Molecular Neuroscience : MN. 19 (1-2): 187–93. PMID 12212779. doi:10.1007/s12031-002-0031-5.
- Jump up^ Mirza NR, Peters D, Sparks RG (2003). “Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists”. CNS Drug Reviews. 9 (2): 159–86. PMID 12847557. doi:10.1111/j.1527-3458.2003.tb00247.x.
- Jump up^ Shekhar A, Potter WZ, Lightfoot J, et al. (August 2008). “Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia”. The American Journal of Psychiatry. 165 (8): 1033–9. PMID 18593778. doi:10.1176/appi.ajp.2008.06091591.
 |
|
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.208.938 |
| Chemical and physical data | |
| Formula | C14H23N3OS |
| Molar mass | 281.42 g/mol |
| 3D model (JSmol) | |
///////Xanomeline, LY 246708, Lumeron, Memcor, ксаномелин , كسانوميلين , 诺美林 , allosteric modulation, Alzheimer’s disease, antipsychotic, muscarinic acetylcholine receptors, schizophrenia,
















