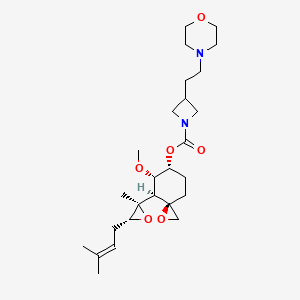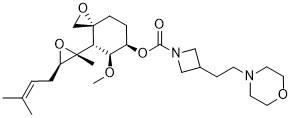
Aclimostat
CAS: 2082752-83-6
Chemical Formula: C26H42N2O6
Molecular Weight: 478.63
Elemental Analysis: C, 65.25; H, 8.85; N, 5.85; O, 20.06
ZGN-1061; ZGN1061; ZGN 1061; Aclimostat,
UNII-X150A3JK8R
X150A3JK8R
(3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3- methylbut-2-en-1-yl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl 3-[2-(morpholin-4-yl)ethyl]azetidine-1-carboxylate
1-Azetidinecarboxylic acid, 3-[2-(4-morpholinyl)ethyl]-, (3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-2-buten-1-yl)-2-oxiranyl]-1-oxaspiro[2.5]oct-6-yl ester
3R,4S,5S,6R)-5-methoxy-4-((2R,3R)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl)-1- oxaspiro[2.5]octan-6-yl 3-(2-morpholinoethyl)azetidine-1-carboxylate
ZAFGEN, PHASE 2, DIABETES
Aclimostat, also known as ZGN-1061, is an anti-diabetic, anti-obesity MetAP2 inhibitor.
Over 1.1 billion people worldwide are reported to be overweight. Obesity is estimated to affect over 90 million people in the United States alone. Twenty-five percent of the population in the United States over the age of twenty is considered clinically obese. While being overweight or obese presents problems (for example restriction of mobility, discomfort in tight spaces such as theater or airplane seats, social difficulties, etc.), these conditions, in particular clinical obesity, affect other aspects of health, i.e., diseases and other adverse health conditions associated with, exacerbated by, or precipitated by being overweight or obese. The estimated mortality from obesity-related conditions in the United States is over 300,000 annually (O’Brien et al. Amer J Surgery (2002) 184:4S-8S; and Hill et al. (1998) Science, 280:1371). [0003] There is no curative treatment for being overweight or obese. Traditional pharmacotherapies for treating an overweight or obese subject, such as serotonin and noradrenergic re-uptake inhibitors, noradrenergic re-uptake inhibitors, selective serotonin re- uptake inhibitors, intestinal lipase inhibitors, or surgeries such as stomach stapling or gastric banding, have been shown to provide minimal short-term benefits or significant rates of relapse, and have further shown harmful side-effects to patients. [0004] MetAP2 encodes a protein that functions at least in part by enzymatically removing the amino terminal methionine residue from certain newly translated proteins such as glyceraldehyde-3-phosphate dehydrogenase (Warder et al. (2008) J. Proteome Res.7:4807). Increased expression of the MetAP2 gene has been historically associated with various forms of cancer. Molecules inhibiting the enzymatic activity of MetAP2 have been identified and have been explored for their utility in the treatment of various tumor types (Wang et al. (2003) Cancer Res.63:7861) and infectious diseases such as microsporidiosis, leishmaniasis, and malaria (Zhang et al. (2002) J. Biomed. Sci.9:34). Notably, inhibition of MetAP2 activity in obese and obese-diabetic animals leads to a reduction in body weight in part by increasing the oxidation of fat and in part by reducing the consumption of food (Rupnick et al. (2002) Proc. Natl. Acad. Sci. USA 99:10730).
[0005] Such MetAP2 inhibitors may be useful as well for patients with excess adiposity and conditions related to adiposity including type 2 diabetes, hepatic steatosis, and
cardiovascular disease (via e.g. ameliorating insulin resistance, reducing hepatic lipid content, and reducing cardiac workload). Accordingly, compounds capable of modulating MetAP2 are needed to address the treatment of obesity and related diseases as well as other ailments favorably responsive to MetAP2 modulator treatment.
Synthesis
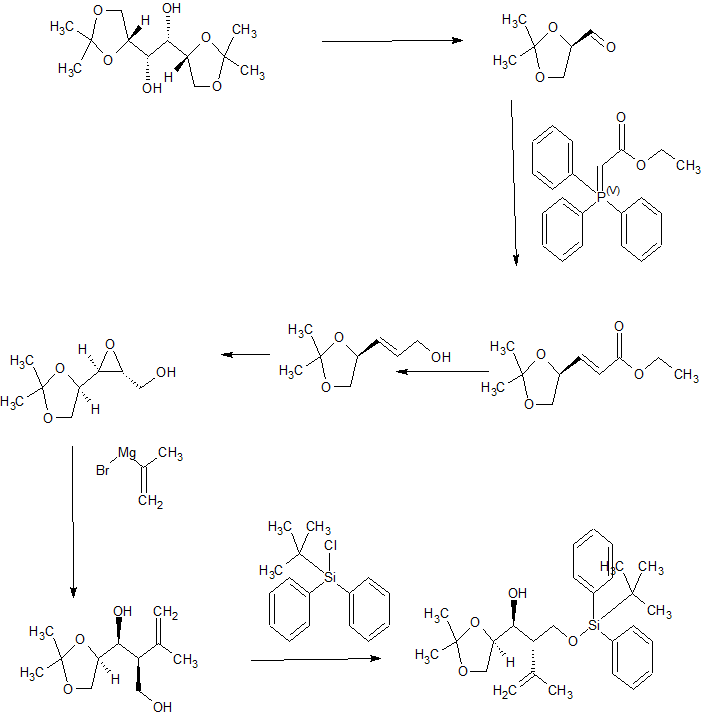
CONTD……………….
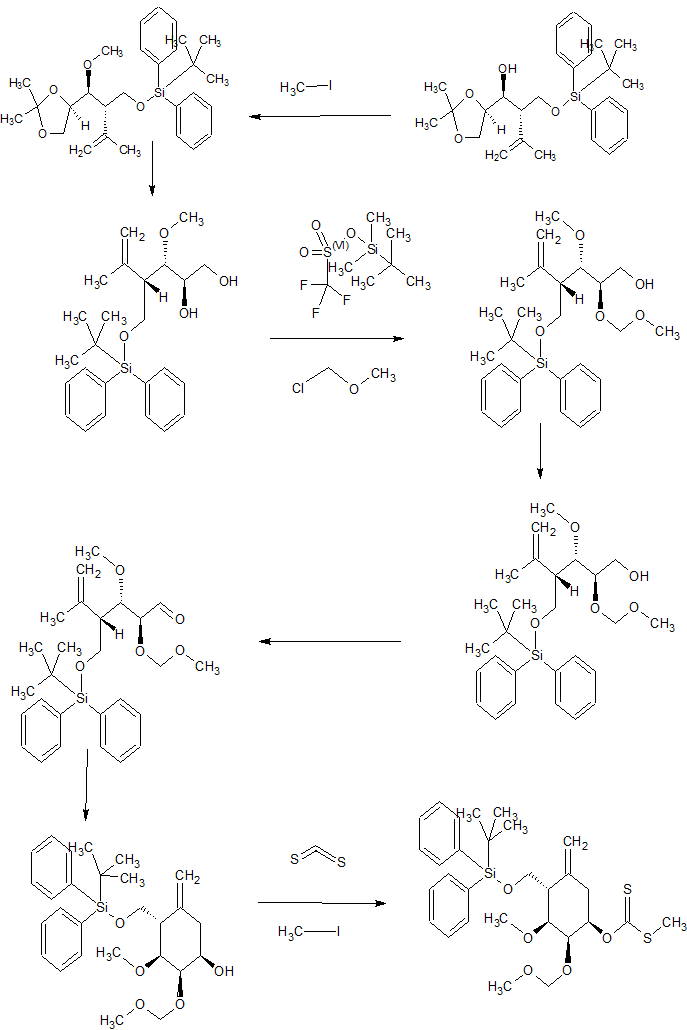
contd………………….
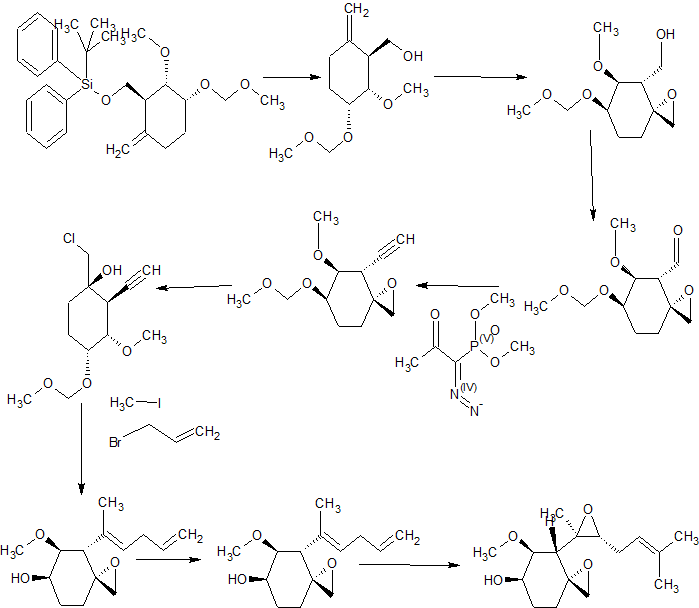
Tetrahedron, 73(30), 4371-4379; 2017
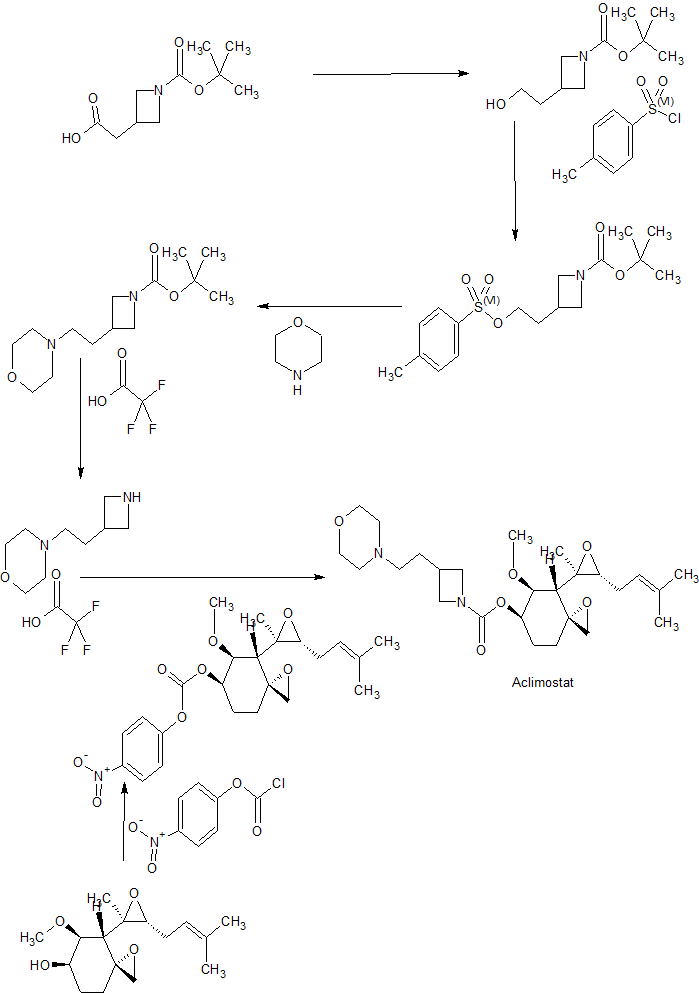
WO 2017027684
PATENT
WO 2017027684
https://patents.google.com/patent/WO2017027684A1/en
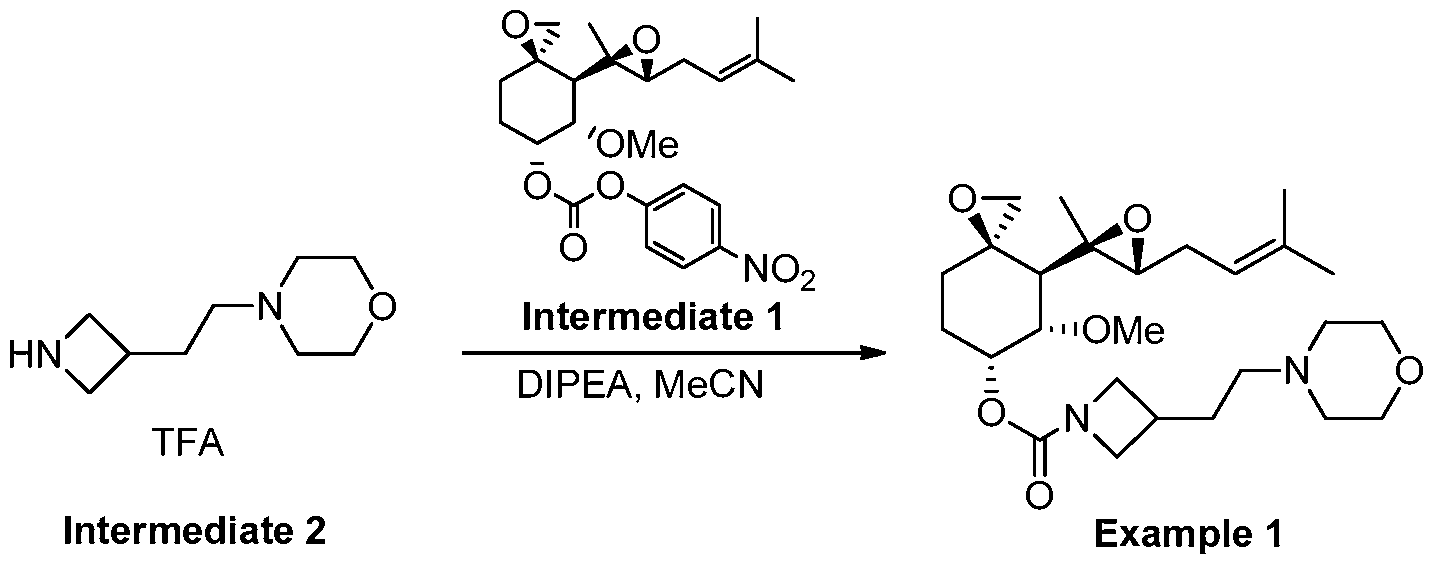
Example 1
(3R,4S,5S,6R)-5-methoxy-4-((2R,3R)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl)-1- oxaspiro[2.5]octan-6-yl 3-(2-morpholinoethyl)azetidine-1-carboxylate
[00312] To a mixture of 4-(2-(azetidin-3-yl)ethyl)morpholine, trifluoroacetate (2.33 g, 3.7 mmol) in CH3CN (150 mL) was added DIPEA (2.9 mL, 17 mmol) drop-wise at 0-5oC. The mixture was then stirred at 0-5oC for 10 min, and carbonate Intermediate 1 (1.3 g, 2.9 mmol) was added to the mixture in portions at 0oC under a N2atmosphere. The reaction mixture was stirred at 25oC for 16 hrs. TLC (PE : EtOAc = 3 : 1) showed that the reaction was complete. The solvent was removed under vacuum below 40oC. The residue was diluted with DCM (60 mL), and the DCM solution was washed with ammonium acetate buffer (pH~4, 15 mL x 2). The combined aqueous layers were back-extracted with DCM (20 mL x 2). The combined organic layers were washed with aq. NaHCO3 solution (15 mL x 2, 5% wt), dried over Na2SO4 and concentrated. Purification by silica gel column chromatography (DCM: MeOH=100: 0~60: 1), followed by preparative HPLC (Method A, H2O (0.1% FA) / CH3CN) gave the title compound (1.15 g) as a light yellow syrup. LC-MS: m/z = 479 [M+H]+; 1H-NMR (400 MHz, CDCl3) δ 5.43 (br, 1H), 5.13 (t, J = 7.6 Hz, 1H), 3.87-4.15 (m, 2H), 3.63-3.65 (m, 4H), 3.52- 3.56 (m, 3H), 3.49 (s, 3H), 2.90 (d, J = 4.4 Hz, 1H), 2.46-2.54 (m, 3H), 2.19-2.36 (m, 7H), 1.97-2.13 (m, 2H), 1.78-1.89 (m, 5H), 1.73 (s, 3H), 1.62 (s, 3H), 1.13 (s, 3H), 0.99 (d, J = 13.6 Hz, 1H).
REFERENCES
1: Malloy J, Zhuang D, Kim T, Inskeep P, Kim D, Taylor K. Single and multiple dose evaluation of a novel MetAP2 inhibitor: Results of a randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes Metab. 2018 Aug;20(8):1878-1884. doi: 10.1111/dom.13305. Epub 2018 Apr 23. PubMed PMID: 29577550; PubMed Central PMCID: PMC6055687.
2: Burkey BF, Hoglen NC, Inskeep P, Wyman M, Hughes TE, Vath JE. Preclinical Efficacy and Safety of the Novel Antidiabetic, Antiobesity MetAP2 Inhibitor ZGN-1061. J Pharmacol Exp Ther. 2018 May;365(2):301-313. doi: 10.1124/jpet.117.246272. Epub 2018 Feb 28. PubMed PMID: 29491038.
//////////////Aclimostat, ZGN-1061, ZAFGEN, PHASE 2, DIABETES
O=C(N1CC(CCN2CCOCC2)C1)O[C@H](CC3)[C@@H](OC)[C@H]([C@@]4(C)O[C@@H]4C/C=C(C)\C)[C@]53CO5