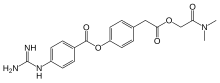
Camostat
- Molecular FormulaC20H22N4O5
- Average mass398.413 Da
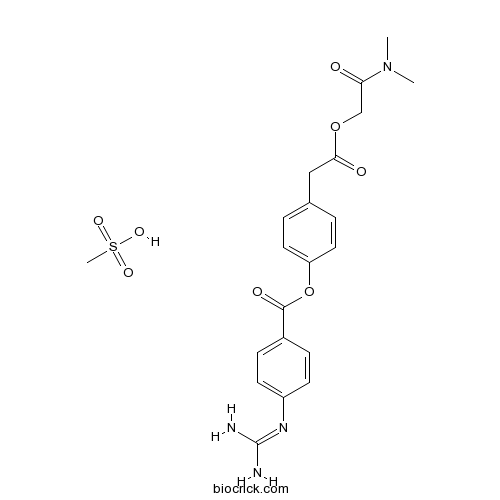
Camostat Mesilateカモスタットメシル酸塩 日局収載 Trypsin-like protease inhibitor CAS 59721-29-8 |
C20H22N4O5.CH4O3S
494.52
MP 194, methanol, diethyl ether, Chemical and Pharmaceutical Bulletin, 2005, vol. 53, 8, pg. 893 – 898
カモスタットメシル酸塩 日局収載
Camostat Mesilate

Dimethylcarbamoylmethyl 4-(4-guanidinobenzoyloxy)phenylacetate monomethanesulfonate
C20H22N4O5▪CH4O3S : 494.52
[59721-29-8]
Launched – 1985, in Japan by Ono for the oral treatment of postoperative reflux esophagitis and chronic pancreatitis.
Camostat mesilate is a synthetic serine protease inhibitor that has been launched in Japan by Ono for the oral treatment of postoperative reflux esophagitis and chronic pancreatitis. It has been demonstrated that the drug has the ability to inhibit proteases such as trypsin, kallikrein, thrombin, plasmin, and C1 esterase, and that it decreases inflammation by directly suppressing the activity of monocytes and pancreatic stellate cells (PSCs).
In 2011, orphan drug designation was received in the U.S. by Stason Pharmaceuticals for the treatment of chronic pancreatitis. In 2017, Kangen Pharmaceuticals acquired KC Specialty Therapeutics (formerly a wholly-owned subsidiary of Stason Pharmaceuticals).
Camostat (INN; development code FOY-305) is a serine protease inhibitor. Serine protease enzymes have a variety of functions in the body, and so camostat has a diverse range of uses. It is used in the treatment of some forms of cancer and is also effective against some viral infections, as well as inhibiting fibrosis in liver or kidney disease or pancreatitis.[1][2][3][4][5] It is approved in Japan for the treatment of pancreatitis.[6][7]
An in vitro study shows that Camostat reduces significantly the infection of Calu-3 lung cells by SARS-CoV-2, the virus responsible for COVID-19.[8]
SYN
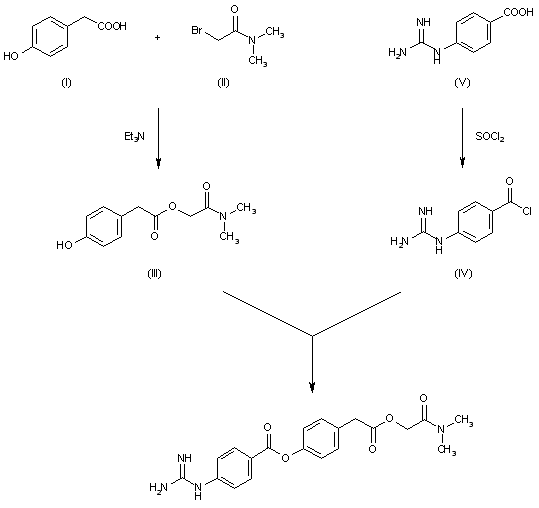
DE 2548886; FR 2289181; GB 1472700; JP 76054530; US 4021472
The reaction of p-hydrophenylacetic acid (I) with N,N-dimethylbromoacetamide (II) by means of triethylamine in reftuxing acetonitrile gives N,N-dimethylcarbamoylmethyl-p-hydroxyphenylacetate (III), which is then condensed with p-guanidinobenzoyl chloride (IV) [obtained from the corresponding acid p-guanidinobenzoic acid (V) and thionyl chloride] in pyridine.
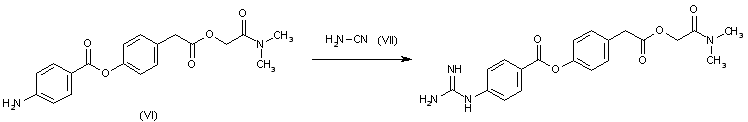
By reaction of N,N-dimethylcarbamoylmethyl-p-(p-aminobenzoyloxy)phenylacetate (VI) with cyanamide (VII).
PATENT
DE 2548886
JP 52089640
JP 54052052
PATENT
CN 104402770
https://patents.google.com/patent/CN104402770A/en
Camostat mesilate, chemical name is 4-(4-guanidine radicals benzoyloxy group) toluylic acid-N, N-dimethyl carbamoyl methyl esters mesylate, be the non-peptide proteinoid enzyme inhibitors of Japanese little Ye medicine Co., Ltd. exploitation, first in January, 1985 go on the market with trade(brand)name Foipan in Japan.Pharmacological evaluation shows: camostat mesilate has very strong restraining effect to trypsinase, kallikrein, Tryptase, zymoplasm, C1 esterase, oral rear kassinin kinin generation system, fibrinolytic system, blood coagulation system and the complement system acting on rapidly body, suppress the exception of the enzymic activity of these systems hyperfunction, thus control the symptom of chronic pancreatitis, alleviating pain, reduce amylase value, the clinical alleviation for chronic pancreatitis acute symptom.In addition, this product is also used for the treatment of diffusivity blood vessel blood coagulation disease.Pharmacological evaluation also finds, camostat mesilate also has the effects such as anticancer, antiviral, and effectively can reduce proteinuria, and play the effect of preliminary conditioning, further research is still underway.Current this product not yet in Discussion on Chinese Listed, also without the report succeeded in developing.
A preparation method for camostat mesilate, comprises the steps:
(1), by 160g methylene dichloride DCM join stirring in reaction vessel, cooling, be cooled to start when 0–10 DEG C to drip 51g 50% dimethylamine agueous solution, drip 30g chloroacetyl chloride simultaneously; Drip process control temp 5–10 DEG C, system pH controls 4-7, at 5–10 DEG C, react 1h after dripping off, reaction process pH controls 5-7, and reaction terminates rear standing 20min, separatory, water layer is with 54g dichloromethane extraction, and organic layer is concentrating under reduced pressure below 80 DEG C, obtains 3-pyrrolidone hydrochloride, crude, 3-pyrrolidone hydrochloride, crude carries out underpressure distillation within 130 DEG C, obtains 3-pyrrolidone hydrochloride distillation product; Output is 31g;
(2), the 3-pyrrolidone hydrochloride of 30.6g, 9g triethylamine TEA, 0.4g sodium bisulfite and 40g p-hydroxyphenylaceticacid p-hydroxyphenylaceticacid drop in order in reaction vessel and carry out stirring at low speed, and then drip the triethylamine of 17.6g, dropping temperature 40-95 DEG C, drip off rear maintenance 80-95 DEG C reaction 3h, after reaction terminates, add aqueous solution of sodium bisulfite (0.05gNaHSO3+90gH2O), add and start more than temperature 70 C, add finishing temperature more than 48 DEG C, after adding, cool, crystal seed is added when 40 DEG C, keep cooling temperature 0-5 DEG C, crystallization 2h, filter after crystallization, filter cake 100g purified water is washed, camostat mesilate crude product is obtained after draining, camostat mesilate crude product, 50mL ethyl acetate are joined heating for dissolving in aqueous solution of sodium bisulfite (0.2g NaHSO3+20g H2O), after having dissolved, cooling crystallization, keep recrystallization temperature 0-5 DEG C, crystallization time 1h, suction filtration after crystallization, filter cake, with 10mL water washing, washs with 20mL ethyl acetate after draining again, again at 60 ± 3 DEG C of drying under reduced pressure 2h after draining, obtain camostat mesilate refined silk, output is about 47g,
(3), the camostat mesilate refined silk of 47g is joined heating for dissolving in 30mL acetonitrile, after dissolving terminates, cooling temperature is to 0-5 DEG C, crystallization 1h, after crystallization terminates, suction filtration, filter cake with 17mL acetonitrile wash, drain, drying under reduced pressure 2h at 60 ± 3 DEG C, obtain camostat mesilate product, output is about 45g.
PATENT
https://patents.google.com/patent/CN104402770B/en
Clip
https://www.pharmaceutical-technology.com/news/german-researchers-covid-19-drug/
German researchers identify potential drug for Covid-19

Scientists at the German Primate Center – Leibniz Institute for Primate Research have found that an existing drug may help treat Covid-19.
As well as Charité – Universitätsmedizin Berlin, the scientists worked with researchers at the University of Veterinary Medicine Hannover Foundation, the BG-Unfallklinik Murnau, the LMU Munich, the Robert Koch Institute and the German Center for Infection Research.
The research aimed to understand the entry of the novel coronavirus, SARS-CoV-2, into host cells, as well as determine approaches to block the process.
Research findings showed that SARS-CoV-2 requires cellular protein TMPRSS2 to enter hosts’ lung cells.
German Primate Center Infection Biology Unit head Stefan Pöhlmann said: “Our results show that SARS-CoV-2 requires the protease TMPRSS2, which is present in the human body, to enter cells. This protease is a potential target for therapeutic intervention.”
CLIP
https://neurosciencenews.com/tmprss2-coronavirus-treatment-15873/
Summary: A clinically proven drug known to block an enzyme essential for the viral entry of Coronavirus into the lungs blocks the COVID 19 (SARS-CoV-2) infection. The drug, Camostat mesilate, is a drug approved in Japan to treat pancreatic inflammation. Results suggest this drug may also protect against COVID 19. Researchers call for further clinical trials.
Viruses must enter cells of the human body to cause disease. For this, they attach to suitable cells and inject their genetic information into these cells. Infection biologists from the German Primate Center – Leibniz Institute for Primate Research in Göttingen, together with colleagues at Charité – Universitätsmedizin Berlin, have investigated how the novel coronavirus SARS-CoV-2 penetrates cells. They have identified a cellular enzyme that is essential for viral entry into lung cells: the protease TMPRSS2. A clinically proven drug known to be active against TMPRSS2 was found to block SARS-CoV-2 infection and might constitute a novel treatment option.
The findings have been published in Cell.
Several coronaviruses circulate worldwide and constantly infect humans, which normally caused only mild respiratory disease. Currently, however, we are witnessing a worldwide spread of a new coronavirus with more than 101,000 confirmed cases and almost 3,500 deaths. The new virus has been named SARS coronavirus-2 and has been transmitted from animals to humans. It causes a respiratory disease called COVID-19 that may take a severe course. The SARS coronavirus-2 has been spreading since December 2019 and is closely related to the SARS coronavirus that caused the SARS pandemic in 2002/2003. No vaccines or drugs are currently available to combat these viruses.
Stopping virus spread
A team of scientists led by infection biologists from the German Primate Centre and including researchers from Charité, the University of Veterinary Medicine Hannover Foundation, the BG-Unfallklinik Murnau, the LMU Munich, the Robert Koch Institute and the German Center for Infection Research, wanted to find out how the new coronavirus SARS-CoV-2 enters host cells and how this process can be blocked. The researchers identified a cellular protein that is important for the entry of SARS-CoV-2 into lung cells. “Our results show that SARS-CoV-2 requires the protease TMPRSS2, which is present in the human body, to enter cells,” says Stefan Pöhlmann, head of the Infection Biology Unit at the German Primate Center. “This protease is a potential target for therapeutic intervention.”
The SARS coronavirus-2 has been spreading since December 2019 and is closely related to the SARS coronavirus that caused the SARS pandemic in 2002/2003. No vaccines or drugs are currently available to combat these viruses. The image is credited to CDC.
Promising drug
Since it is known that the drug camostat mesilate inhibits the protease TMPRSS2, the researchers have investigated whether it can also prevent infection with SARS-CoV-2. “We have tested SARS-CoV-2 isolated from a patient and found that camostat mesilate blocks entry of the virus into lung cells,” says Markus Hoffmann, the lead author of the study. Camostat mesilate is a drug approved in Japan for use in pancreatic inflammation. “Our results suggest that camostat mesilate might also protect against COVID-19,” says Markus Hoffmann. “This should be investigated in clinical trials.”
References
- ^ Okuno, M.; Kojima, S.; Akita, K.; Matsushima-Nishiwaki, R.; Adachi, S.; Sano, T.; Takano, Y.; Takai, K.; Obora, A.; Yasuda, I.; Shiratori, Y.; Okano, Y.; Shimada, J.; Suzuki, Y.; Muto, Y.; Moriwaki, Y. (2002). “Retinoids in liver fibrosis and cancer”. Frontiers in Bioscience. 7 (4): d204-18. doi:10.2741/A775. PMID 11779708.
- ^ Hsieh, H. P.; Hsu, J. T. (2007). “Strategies of development of antiviral agents directed against influenza virus replication”. Current Pharmaceutical Design. 13 (34): 3531–42. doi:10.2174/138161207782794248. PMID 18220789.
- ^ Kitamura, K.; Tomita, K. (2012). “Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension”. Clinical and Experimental Nephrology. 16 (1): 44–8. doi:10.1007/s10157-011-0506-1. PMID 22038264.
- ^ Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion Jr, R.; Nunneley, J. W.; Barnard, D.; Pöhlmann, S.; McKerrow, J. H.; Renslo, A. R.; Simmons, G. (2015). “Protease inhibitors targeting coronavirus and filovirus entry”. Antiviral Research. 116: 76–84. doi:10.1016/j.antiviral.2015.01.011. PMC 4774534. PMID 25666761.
- ^ Ueda, M.; Uchimura, K.; Narita, Y.; Miyasato, Y.; Mizumoto, T.; Morinaga, J.; Hayata, M.; Kakizoe, Y.; Adachi, M.; Miyoshi, T.; Shiraishi, N.; Kadowaki, D.; Sakai, Y.; Mukoyama, M.; Kitamura, K. (2015). “The serine protease inhibitor camostat mesilate attenuates the progression of chronic kidney disease through its antioxidant effects”. Nephron. 129 (3): 223–32. doi:10.1159/000375308. PMID 25766432.
- ^ “Covid-19 potential drug identified by German researchers”. www.pharmaceutical-technology.com. Retrieved 2020-03-14.
- ^ “Camostat”. drugs.com.
- ^ Hoffman, Markus (2020-03-05). “SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor”. Cell. Retrieved 2020-03-05.
External links
- Kunze H, Bohn E (May 1983). “Effects of the serine protease inhibitors FOY and FOY 305 on phospholipase A1 (EC 3.1.1.32) activity in rat – liver lysosomes”. Pharmacol Res Commun. 15 (5): 451–9. doi:10.1016/S0031-6989(83)80065-4. PMID 6412250.
- Göke B, Stöckmann F, Müller R, Lankisch PG, Creutzfeldt W (1984). “Effect of a specific serine protease inhibitor on the rat pancreas: systemic administration of camostate and exocrine pancreatic secretion”. Digestion. 30 (3): 171–8. doi:10.1159/000199102. PMID 6209186.
 |
|
| Clinical data | |
|---|---|
| Trade names | Foipan |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H22N4O5 |
| Molar mass | 398.419 g·mol−1 |
| 3D model (JSmol) | |
/////////////Camostat, SARS-CoV-2, COVID-19, coronavirus, SARS-CoV-2, COVID-19, FOY305, FOY-S980, カモスタットメシル酸塩 日局収載 , Japan, Ono, oral treatment of postoperative reflux esophagitis, chronic pancreatitis.
CN(C)C(=O)COC(=O)CC1=CC=C(C=C1)OC(=O)C2=CC=C(C=C2)N=C(N)N.CS(=O)(=O)O















