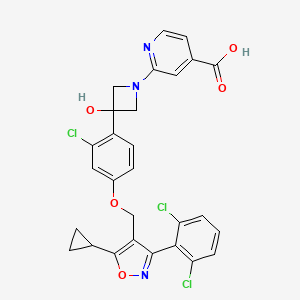
CILOFEXOR
| 586.8 g/mol |
1418274-28-8
GS-9674, Cilofexor (GS(c)\9674)
UNII-YUN2306954
YUN2306954
2-[3-[2-chloro-4-[[5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazol-4-yl]methoxy]phenyl]-3-hydroxyazetidin-1-yl]pyridine-4-carboxylic acid
Cilofexor is under investigation in clinical trial NCT02943447 (Safety, Tolerability, and Efficacy of Cilofexor in Adults With Primary Biliary Cholangitis Without Cirrhosis).
Cilofexor (GS-9674) is a potent, selective and orally active nonsteroidal FXR agonist with an EC50 of 43 nM. Cilofexor has anti-inflammatory and antifibrotic effects. Cilofexor has the potential for primary sclerosing cholangitis (PSC) and nonalcoholic steatohepatitis (NASH) research.
Gilead , following a drug acquisition from Phenex , is developing cilofexor tromethamine (formerly GS-9674), the lead from a program of farnesoid X receptor (FXR; bile acid receptor) agonists, for the potential oral treatment of non-alcoholic steatohepatitis (NASH), primary biliary cholangitis/cirrhosis (PBC) and primary sclerosing cholangitis. In March 2019, a phase III trial was initiated for PSC; at that time, the trial was expected to complete in August 2022.
PATENT
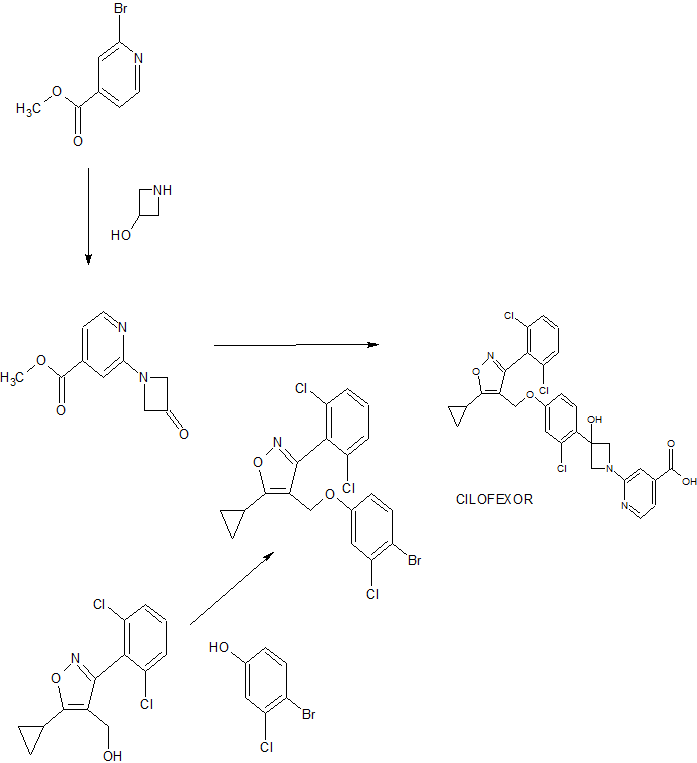
Product case WO2013007387 , expiry EU in 2032 and in the US in 2034.
https://patents.google.com/patent/WO2013007387A1/en
WO2020150136 claiming 2,6-dichloro-4-fluorophenyl compounds.
PATENT
Novel crystalline forms of cilofexor as FXR agonists useful for treating nonalcoholic steatohepatitis. Gilead , following a drug acquisition from Phenex , is developing cilofexor tromethamine (formerly GS-9674), the lead from a program of farnesoid X receptor (FXR; bile acid receptor) agonists, for the potential oral treatment of non-alcoholic steatohepatitis (NASH), primary biliary cholangitis/cirrhosis (PBC) and primary sclerosing cholangitis. In March 2019, a phase III trial was initiated for PSC; at that time, the trial was expected to complete in August 2022. Family members of the cilofexor product case WO2013007387 , expire in the EU in 2032 and in the US in 2034.
solid forms of compounds that bind to the NR1H4 receptor (FXR) and act as agonists or modulators of FXR. The disclosure further relates to the use of the solid forms of such compounds for the treatment and/or prophylaxis of diseases and/or conditions through binding of said nuclear receptor by said compounds.
[0004] Compounds that bind to the NR1H4 receptor (FXR) can act as agonists or modulators of FXR. FXR agonists are useful for the treatment and/or prophylaxis of diseases and conditions through binding of the NR1H4 receptor. One such FXR agonist is the compound of Formula I:
I.
[0005] Although numerous FXR agonists are known, what is desired in the art are physically stable forms of the compound of Formula I, or pharmaceutically acceptable salt thereof, with desired properties such as good physical and chemical stability, good aqueous solubility and good bioavailability. For example, pharmaceutical compositions are desired that address
challenges of stability, variable pharmacodynamics responses, drug-drug interactions, pH effect, food effects, and oral bioavailability.
[0006] Accordingly, there is a need for stable forms of the compound of Formula I with suitable chemical and physical stability for the formulation, therapeutic use, manufacturing, and storage of the compound.
[0007] Moreover, it is desirable to develop a solid form of Formula I that may be useful in the synthesis of Formula I. A solid form, such as a crystalline form of a compound of Formula I may be an intermediate to the synthesis of Formula F A solid form may have properties such as bioavailability, stability, purity, and/or manufacturability at certain conditions that may be suitable for medical or pharmaceutical uses.
| Description | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 & Target |
EC50: 43 nM (FXR)[1] |
||||||||||||||||||||||||||||||||||||||||||||||||||
| In Vivo |
Cilofexor (GS-9674; 30 mg/kg; oral gavage; once daily; for 10 weeks; male Wistar rats) treatment significantly increases Fgf15 expression in the ileum and decreased Cyp7a1 in the liver in nonalcoholic steatohepatitis (NASH) rats. Liver fibrosis and hepatic collagen expression are significantly reduced. Cilofexor also significantly reduces hepatic stellate cell (HSC) activation and significantly decreases portal pressure, without affecting systemic hemodynamics[3].
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical Trial |
|
| Patent ID | Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2019142814 | Novel FXR (NR1H4) binding and activity modulating compounds | 2019-01-15 | |
| US2019055273 | ACYCLIC ANTIVIRALS | 2017-03-09 | |
| US10220027 | FXR (NR1H4) binding and activity modulating compounds | 2017-10-13 | |
| US10071108 | Methods and pharmaceutical compositions for the treatment of hepatitis b virus infection | 2018-02-19 | |
| US2018000768 | INTESTINAL FXR AGONISM ENHANCES GLP-1 SIGNALING TO RESTORE PANCREATIC BETA CELL FUNCTIONS | 2017-09-06 |
| Patent ID | Title | Submitted Date | Granted Date |
|---|---|---|---|
| US9820979 | NOVEL FXR (NR1H4) BINDING AND ACTIVITY MODULATING COMPOUNDS | 2016-12-05 | |
| US9539244 | NOVEL FXR (NR1H4) BINDING AND ACTIVITY MODULATING COMPOUNDS | 2015-08-12 | 2015-12-03 |
| US9895380 | METHODS AND PHARMACEUTICAL COMPOSITIONS FOR THE TREATMENT OF HEPATITIS B VIRUS INFECTION | 2014-09-10 | 2016-08-04 |
| US2017355693 | FXR (NR1H4) MODULATING COMPOUNDS | 2017-06-12 | |
| US2016376279 | FXR AGONISTS AND METHODS FOR MAKING AND USING | 2016-09-12 |
| Patent ID | Title | Submitted Date | Granted Date |
|---|---|---|---|
| US9139539 | NOVEL FXR (NR1H4) BINDING AND ACTIVITY MODULATING COMPOUNDS | 2012-07-12 | 2014-08-07 |
| US2018133203 | METHODS OF TREATING LIVER DISEASE | 2017-10-27 |
ClinicalTrials.gov
| CTID | Title | Phase | Status | Date |
|---|---|---|---|---|
| NCT03890120 | Safety, Tolerability, and Efficacy of Cilofexor in Non-Cirrhotic Adults With Primary Sclerosing Cholangitis | Phase 3 | Recruiting | 2020-08-31 |
| NCT02781584 | Safety, Tolerability, and Efficacy of Selonsertib, Firsocostat, and Cilofexor in Adults With Nonalcoholic Steatohepatitis (NASH) | Phase 2 | Recruiting | 2020-08-13 |
| NCT03987074 | Safety, Tolerability, and Efficacy of Monotherapy and Combination Regimens in Adults With Nonalcoholic Steatohepatitis (NASH) | Phase 2 | Completed | 2020-07-29 |
| NCT02943460 | Safety, Tolerability, and Efficacy of Cilofexor in Adults With Primary Sclerosing Cholangitis Without Cirrhosis | Phase 2 | Completed | 2020-06-09 |
| NCT02943447 | Safety, Tolerability, and Efficacy of Cilofexor in Adults With Primary Biliary Cholangitis Without Cirrhosis | Phase 2 | Completed | 2020-02-11 |
ClinicalTrials.gov
| CTID | Title | Phase | Status | Date |
|---|---|---|---|---|
| NCT03449446 | Safety and Efficacy of Selonsertib, Firsocostat, Cilofexor, and Combinations in Participants With Bridging Fibrosis or Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis (NASH) | Phase 2 | Completed | 2019-12-24 |
| NCT02854605 | Evaluating the Safety, Tolerability, and Efficacy of GS-9674 in Participants With Nonalcoholic Steatohepatitis (NASH) | Phase 2 | Completed | 2019-01-29 |
| NCT02808312 | Pharmacokinetics and Pharmacodynamics of GS-9674 in Adults With Normal and Impaired Hepatic Function | Phase 1 | Completed | 2018-10-30 |
| NCT02654002 | Study in Healthy Volunteers to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of GS-9674, and the Effect of Food on GS-9674 Pharmacokinetics and Pharmacodynamics | Phase 1 | Completed | 2016-07-27 |
EU Clinical Trials Register
| EudraCT | Title | Phase | Status | Date |
|---|---|---|---|---|
| 2019-000204-14 | A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety, Tolerability, and Efficacy of Cilofexor in Non-Cirrhotic Subjects with Primary Sclerosing Cholangitis | Phase 3 | Restarted, Ongoing | 2019-09-11 |
| 2016-002496-10 | A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety, Tolerability, and Efficacy of GS-9674 in Subjects with Nonalcoholic Steatohepatitis (NASH) | Phase 2 | Completed | 2017-02-21 |
| 2016-002443-42 | A Phase 2, Randomized, Double-Blind, Placebo Controlled Study Evaluating the Safety, Tolerability, and Efficacy of GS-9674 in Subjects with Primary Biliary Cholangitis Without Cirrhosis | Phase 2 | Completed | 2017-01-09 |
| 2016-002442-23 | A Phase 2, Randomized, Double-Blind, Placebo Controlled Study Evaluating the Safety, Tolerability, and Efficacy of GS-9674 in Subjects with Primary Sclerosing Cholangitis Without Cirrhosis | Phase 2 | Completed | 2017-01-09 |
///////////CILOFEXOR, Cilofexor (GS(c)\9674), GS-9674, phase 3
C1CC1C2=C(C(=NO2)C3=C(C=CC=C3Cl)Cl)COC4=CC(=C(C=C4)C5(CN(C5)C6=NC=CC(=C6)C(=O)O)O)Cl