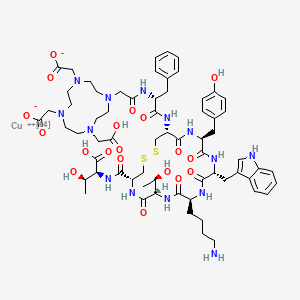
Copper Cu 64 dotatate
銅(Cu64)ドータテート;
UNII-N3858377KC
N3858377KC
Copper 64-DOTA-tate
Copper Cu-64 dotatate
Copper dotatate Cu-64
Diagnostic (neuroendocrine tumors), Radioactive agent
| Formula |
C65H86CuN14O19S2. 2H
|
|---|---|
| CAS: |
1426155-87-4
|
| Mol weight |
1497.1526
|
FDA APPROVED 2020. 2020/9/3. Detectnet
2-[4-[2-[[(2R)-1-[[(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-4-[[(1S,2R)-1-carboxy-2-hydroxypropyl]carbamoyl]-7-[(1R)-1-hydroxyethyl]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicos-19-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]-10-(carboxylatomethyl)-7-(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetate;copper-64(2+)
Copper Cu 64 dotatate, sold under the brand name Detectnet, is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adults.[1]
Common side effects include nausea, vomiting and flushing.[2]
It was approved for medical use in the United States in September 2020.[1][2]
History
The U.S. Food and Drug Administration (FDA) approved copper Cu 64 dotatate based on data from two trials that evaluated 175 adults.[3]
Trial 1 evaluated adults, some of whom had known or suspected NETs and some of whom were healthy volunteers.[3] The trial was conducted at one site in the United States (Houston, TX).[3] Both groups received copper Cu 64 dotatate and underwent PET scan imaging.[3] Trial 2 data came from the literature-reported trial of 112 adults, all of whom had history of NETs and underwent PET scan imaging with copper Cu 64 dotatate.[3] The trial was conducted at one site in Denmark.[3] In both trials, copper Cu 64 dotatate images were compared to either biopsy results or other images taken by different techniques to detect the sites of a tumor.[3] The images were read as either positive or negative for presence of NETs by three independent image readers who did not know participant clinical information.[3]
PATENT
https://patents.google.com/patent/WO2013029616A1/en
PATENT
https://patents.google.com/patent/US20140341807
-
Known imaging techniques with tremendous importance in medical diagnostics are positron emission tomography (PET), computed tomography (CT), magnetic resonance imaging (MRI), single photon computed tomography (SPECT) and ultrasound (US). Although today’s imaging technologies are well developed they rely mostly on non-specific, macroscopic, physical, physiological, or metabolic changes that differentiate pathological from normal tissue.
- [0003]
Targeting molecular imaging (MI) has the potential to reach a new dimension in medical diagnostics. The term “targeting” is related to the selective and highly specific binding of a natural or synthetic ligand (binder) to a molecule of interest (molecular target) in vitro or in vivo.
- [0004]
MI is a rapidly emerging biomedical research discipline that may be defined as the visual representation, characterization and quantification of biological processes at the cellular and sub-cellular levels within intact living organisms. It is a novel multidisciplinary field, in which the images produced reflect cellular and molecular pathways and in vivo mechanism of disease present within the context of physiologically authentic environments rather than identify molecular events responsible for disease.
- [0005]
Several different contrast-enhancing agents are known today and their unspecific or non-targeting forms are already in clinical routine. Some examples listed below are reported in literature.
- [0006]
For example, Gd-complexes could be used as contrast agents for MRI according to “Contrast Agents I” by W. Krause (Springer Verlag 2002, page one and following pages). Furthermore, superparamagnetic particles are another example of contrast-enhancing units, which could also be used as contrast agents for MRI (Textbook of Contrast Media, Superparamagnetic Oxides, Dawson, Cosgrove and Grainger Isis Medical Media Ltd, 1999, page 373 and following pages). As described in Contrast Agent II by W. Krause (Springer Verlag 2002, page 73 and following pages), gas-filled microbubbles could be used in a similar way as contrast agents for ultrasound. Moreover “Contrast Agents II” by W. Krause (Springer Verlag, 2002, page 151 and following pages) reports the use of iodinated liposomes or fatty acids as contrast agents for X-Ray imaging.
- [0007]
Contrast-enhancing agents that can be used in functional imaging are mainly developed for PET and SPECT.
- [0008]
The application of radiolabelled bioactive peptides for diagnostic imaging is gaining importance in nuclear medicine. Biologically active molecules which selectively interact with specific cell types are useful for the delivery of radioactivity to target tissues. For example, radiolabelled peptides have significant potential for the delivery of radionuclides to tumours, infarcts, and infected tissues for diagnostic imaging and radiotherapy.
- [0009]
DOTA (1,4,7,10-tetrakis(carboxymethyl)-1,4,7,10tetraazacyclododecane) and its derivatives constitute an important class of chelators for biomedical applications as they accommodate very stably a variety of di- and trivalent metal ions. An emerging area is the use of chelator conjugated bioactive peptides for labeling with radiometals in different fields of diagnostic and therapeutic nuclear oncology.
- [0010]
There have been several reports in recent years on targeted radiotherapy with radiolabeled somatostatin analogs.
- [0011]
US2007/0025910A1 discloses radiolabled somatostatin analogs primarily based on the ligand DOTA-TOC. The radionucleotide can be (64)Copper and the somatostatin analog may be octreotide, lanreotide, depreotide, vapreotide or derivatives thereof. The compounds of US2007/0025910A1 are useful in radionucleotide therapy of tumours.
- [0012]
US2007/0025910A1 does not disclose (64)Cu-DOTA-TATE. DOTA-TATE and DOTA-TOC differ clearly in affinity for the 5 known somatostatin receptors (SST1-SST2). Accordingly, the DOTA-TATE has a 10-fold higher affinity for the SST2 receptor, the receptor expressed to the highest degree on neuroendocrine tumors. Also the relative affinity for the other receptor subtypes are different. Furthermore, since 177Lu-DOTATATE is used for radionuclide therapy, only 64Cu-DOTATATE and not 64Cu-DOTATOC can be used to predict effect of such treatment by a prior PET scan.
- [0013]
There exists a need for further peptide-based compounds having utility for diagnostic imaging techniques, such as PET.
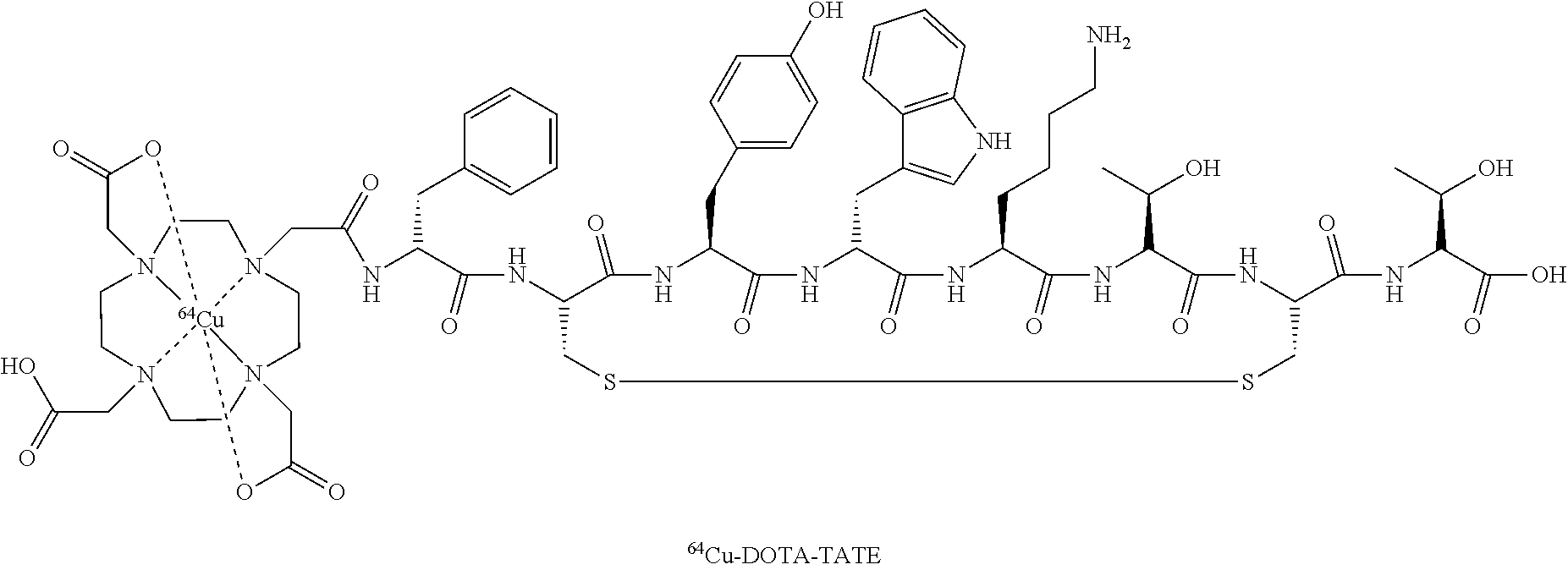
- EXAMPLE
- [0033]
Preparation of “Cu-Dotatate-DOTA-TATE
- [0034]
64Cu was produced using a GE PETtrace cyclotron equipped with a beamline. The 64Cu was produced via the 64Ni (p,n) 64Cu reaction using a solid target system consisting of a water cooled target mounted on the beamline. The target consisted of 64Ni metal (enriched to >99%) electroplated on a silver disc backing. For this specific type of production a proton beam with the energy of 16 MeV and a beam current of 20 uA was used. After irradiation the target was transferred to the laboratory for further chemical processing in which the 64Cu was isolated using ion exchange chromatography. Final evaporation from aq. HCl yielded 2-6 GBq of 64Cu as 64CuCl2 (specific activity 300-3000 TBq/mmol; RNP >99%). The labeling of 64Cu to DOTA-TATE was performed by adding a sterile solution of DOTA-TATE (0.3 mg) and Gentisic acid (25 mg) in aq Sodium acetate (1 ml; 0.4M, pH 5.0) to a dry vial containing 64CuCl2 (˜1 GBq). Gentisic acid was added as a scavenger to reduce the effect of radiolysis. The mixture was left at ambient temperature for 10 minutes and then diluted with sterile water (1 ml). Finally, the mixture was passed through a 0.22 μm sterile filter (Millex GP, Millipore). Radiochemical purity was determined by RP-HPLC and the amount of unlabeled 64Cu2+ was determined by thin-layer chromatography. All chemicals were purchased from Sigma-Aldrich unless specified otherwise. DOTA-Tyr3-Octreotate (DOTA-TATE) was purchased from Bachem (Torrance, Calif.). Nickel-64 was purchased in +99% purity from Campro Scientific Gmbh. All solutions were made using Ultra pure water (<0.07 μSimens/cm). Reversed-phase high pressure liquid chromatography was performed on a Waters Alliance 2795 Separations module equipped with at Waters 2489 UV/Visible detector and a Caroll Ramsey model 105 S-1 radioactivity detector—RP-HPLC column was Luna C18, HST, 50×2 mm, 2.5 μm, Phenomenex. The mobile phase was 5% aq. acetonitrile (0.1% TFA) and 95% aq. acetonitrile (0.1% TFA).
- [0035]
Thin layer chromatography was performed with a Raytest MiniGita Star TLC-scanner equipped with a Beta-detector. The eluent was 50% aq methanol and the TLC-plate was a Silica60 on Al foil (Fluka). Ion exchange chromatography was performed on a Dowex 1×8 resin (Chloride-form, 200-400 mesh).
References
- ^ Jump up to:a b “FDA approval letter” (PDF). 3 September 2020. Retrieved 5 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b “RadioMedix and Curium Announce FDA Approval of Detectnet (copper Cu 64 dotatate injection) in the U.S.” (Press release). Curium. 8 September 2020. Retrieved 9 September 2020 – via GlobeNewswire.
- ^ Jump up to:a b c d e f g h “Drug Trials Snapshots: Detectnet”. U.S. Food and Drug Administration (FDA). 3 September 2020. Retrieved 10 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
- “Copper dotatate Cu-64”. Drug Information Portal. U.S. National Library of Medicine.
- “copper Cu 64 dotatate injection safety data sheet” (PDF). Curium US LLC. 15 March 2020.



The FDA has approved copper Cu 64 dotatate injection (Detectnet) for the localization of somatostatin receptor–positive neuroendocrine tumors (NETs), according to an announcement from RadioMedix Inc. and Curium Pharma.1
The positron emission tomography (PET) diagnostic agent is anticipated to launch immediately, according to Curium. Doses will be accessible through several nuclear pharmacies or through the nuclear medicine company.
“Detectnet brings an exciting advancement in the diagnosis of NETs for healthcare providers, patients, and their caregivers,” Ebrahim Delpassand MD, CEO of RadioMedix, stated in a press release. “The phase 3 results demonstrate the clinical sensitivity and specificity of Detectnet which will provide a great aid to clinicians in developing an accurate treatment approach for their [patients with] NETs.”
Copper Cu 64 dotatate adheres to somatostatin receptors with highest affinity for subtype 2 receptors (SSTR2). Specifically, the agent binds to somatostatin receptor–expressing cells, including malignant neuroendocrine cells; these cells overexpress SSTR2. The agent is a positron-producing radionuclide that possesses an emission yield that permits PET imaging.
“Perhaps most exciting is that the 12.7-hour half-life allows Detectnet to be produced centrally and shipped to sites throughout the United States,” added Delpassand. “This will help alleviate shortages or delays that have been experienced with other somatostatin analogue PET agents.”
Two single-center, open-label studies confirmed the efficacy of the diagnostic agent, according to Curium.2 In Study 1, investigators conducted a prospective analysis of 63 patients, which included 42 patients with known or suspected NETs according to histology, conventional imaging, or clinical evaluations, and 21 healthy volunteers. The majority of the participants, or 88% (n = 37) had a history of NETs at the time that they underwent imaging. Just under half of patients (44%; n = 28) were men and the majority were white (86%). Moreover, patients had a mean age of 54 years.
Images produced by the PET agent were interpreted to be either positive or negative for NET via 3 independent readers who had been blinded to the clinical data and other imaging information. Moreover, the results from the diagnostic agent were compared with a composite reference standard that was comprised of 1 oncologist’s blinded evaluation of patient diagnosis based on available histopathology results, reports of conventional imaging that had been done within 8 weeks before the PET imaging, as well as clinical and laboratory findings, which involved chromogranin A and serotonin levels.
Additionally, the percentage of patients who tested positive for disease via composite reference as well as through PET imaging was used to quantify positive percent agreement. Conversely, the percentage of participants who did not have disease per composite reference and who were determined to be negative for disease per PET imaging was used to quantify negative percent agreement.
Results showed that the percent reader agreement for positive detection in 62 scans was 91% (95% CI, 75-98) and negative detection was 97% (95% CI, 80-99). For reader 2, these percentages were 91% (95% CI, 75-98) and 80% (95% CI, 61-92), respectively, for 63 scans. Lastly, the percent reader agreement for reader 3 in 63 scans was 91% (95% CI, 75-98) positive and 90% (95% CI, 72-97) negative.
Study 2 was a retrospective analysis in which investigators examined published findings collected from 112 patients; 63 patients were male, while 43 were female. The mean age of patients included in the analysis was 62 years. All patients had a known history of NETs. Results demonstrated similar performance with the PET imaging agent.
In both safety and efficacy trials, a total of 71 patients were given a single dose of the diagnostic agent; the majority of these patients had known or suspected NETs and 21 were healthy volunteers. Adverse reactions such as nausea, vomiting, and flushing were reported at a rate of less than 2%. In all clinical experience that has been published, a total of 126 patients with a known history of NETs were given a single dose of the PET diagnostic agent. A total of 4 patients experienced nausea immediately after administration.
“Curium is excited to bring the first commercially available Cu 64 diagnostic agent to the US market,” Dan Brague, CEO of Curium, North America, added in the release. “Our unique production capabilities and distribution network allow us to deliver to any nuclear pharmacy, hospital, or imaging center its full dosing requirements first thing in the morning, to provide scheduling flexibility to the institution and its patients. We look forward to joining with healthcare providers and our nuclear pharmacy partners to bring this highly efficacious agent to the market.”
References
1. RadioMedix and Curium announce FDA approval of Detectnet (copper Cu 64 dotatate injection) in the US. News release. RadioMedix Inc and Curium. September 8, 2020. Accessed September 9, 2020. https://bit.ly/3m6iC0q.
2. Detectnet. Prescribing information. Curium Pharma; 2020. Accessed September 9, 2020. https://bit.ly/32eZxS3.
| Clinical data | |
|---|---|
| Trade names | Detectnet |
| Routes of administration |
Intravenous |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C65H88CuN14O19S2 |
| Molar mass | 1497.16 g·mol−1 |
| 3D model (JSmol) | |
///////////////Copper Cu 64 dotatate, 銅(Cu64)ドータテート , FDA 2020, 2020 APPROVALS, Diagnostic, neuroendocrine tumors, Radioactive agent,
CC(C1C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)CN6CCN(CCN(CCN(CC6)CC(=O)[O-])CC(=O)[O-])CC(=O)O)C(=O)NC(C(C)O)C(=O)O)O.[Cu+2]