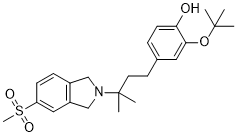
CT-1812
Elayta
Condition(s): Alzheimer’s Disease
U.S. FDA Status: Alzheimer’s Disease (Phase 2)
Company: Cognition Therapeutics Inc.
CAS: 1802632-22-9
Chemical Formula: C24H33NO4S
Molecular Weight: 431.591
2-(tert-butoxy)-4-(3-methyl-3-(5-(methylsulfonyl)isoindolin-2-yl)butyl)phenol
Phenol, 4-[3-[1,3-dihydro-5-(methylsulfonyl)-2H-isoindol-2-yl]-3-methylbutyl]-2-(1,1-dimethylethoxy)-
- Originator Cognition Therapeutics
- Class Antidementias; Neuroprotectants; Nootropics; Small molecules
- Mechanism of Action Sigma-2 receptor antagonists
- Phase II Alzheimer’s disease
- Phase I Cognition disorders
- 21 Feb 2019 Cognition Therapeutics receives patent for a composition of matter patent covering Elayta™ in Europe
- 19 Feb 2019 Pharmacokinetics and adverse events data from a phase I trial in Cognition disorders released by Cognition Therapeutics
- 22 Oct 2018 CTP push 289675: Updated KDM, forwarded USA line from PI/II to PII
CT-1812 is a first-in-class, orally available sigma-2/PGRMC1 antagonist (alpha beta oligomer receptor antagonist), is being developed by Cognition. sCT-1812 is a novel therapeutic candidate for Alzheimer’s disease
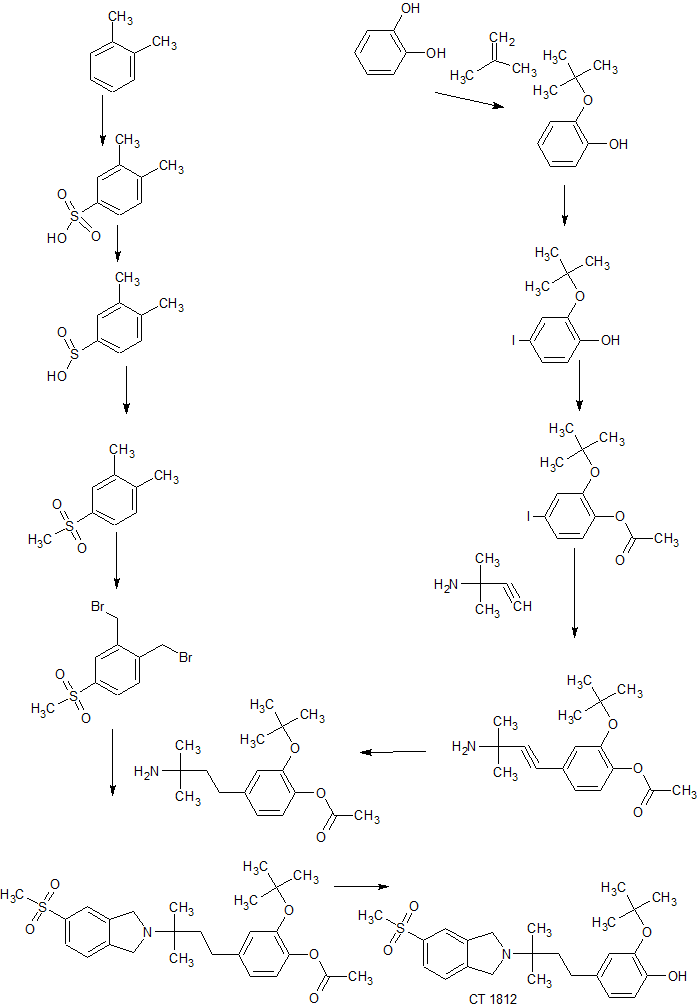
SYN
BACKGROUND
CT1812 is a small-molecule antagonist of the sigma2 receptor, also known as the progesterone receptor membrane component 1. The rationale behind this therapeutic approach is that ligands for the sigma2/PGRMC1 receptor will compete with oligomeric Aβ binding to this receptor and thus interfere with Aβ-induced synaptic toxicity. CT1812 grew out of screening programs at Cognition Therapeutics. Company scientists have reported that compounds in this series not only block binding of a range of different Aβ species to this receptor but also displace it when applied after Aβ has bound (Dec 2014 conference news).
The structure of CT1812 has not been disclosed, but similar compounds in the series have been reported to enter the brain, occupy up to 80 percent of sigma2/PGRMC1 receptors, and restore behavioral deficits in APP transgenic mice (Izzo et al., 2014; Izzo et al., 2014).
FINDINGS
From September 2015 to May 2016, Cognition Therapeutics ran a Phase 1 trial in 80 healthy volunteers aged 18 to 75 in Melbourne, Australia; target enrollment was originally listed as 114. Single-ascending-dose administration was followed by multiple ascending doses given once daily for two weeks. The dose range in this trial spanned 10 to 650 mg; if this would not generate data to set a maximum tolerated dose, doses up to 1,350 mg were to be tried. Outcome measures included safety, tolerability, plasma pharmacokinetics, and CSF CT1812 concentration. At the 2016 and 2017 AAIC conferences, company scientists reported that single doses up to 1,120 mg were given, as were multiple doses of up to 840 mg in young and up to 560 mg in elderly volunteers. The drug was reported to be well-tolerated, with suitable pharmacokinetics, sufficient brain penetrance and target exposure, and minimal drug-drug interactions affecting cytochrome P450 activity (Catalano et al., 2016; Catalano et al., 2017).
From September 2016 to August 2017, Cognition Therapeutics ran a Phase 1/2 trial at four sites in Australia, enrolling 19 participants with mild to moderate Alzheimer’s disease supported by a recent MRI. It compared a four-week course of 90, 280, or 560 mg of CT 1812 to placebo, taken once daily, on safety and tolerability parameters. At the subsequent CTAD conference, Elayta was reported to have been generally safe and well tolerated, though there were four cases of lymphocytopenia. Exploratory measures such as ADAS-Cog14, verbal or category fluency tests recorded no difference between groups, but exploratory biomarker analyses yielded possible signals of synapse protection (Dec 2017 conference news).
In April 2018, a Phase 1/2 study started enrolling 21 people whose mild to moderate AD was confirmed by amyloid PET or CSF testing. Conducted at Yale University School of Medicine and dubbed COG0105 or SPARC, this trial will compare a six-month course of 100 or 300 mg of Elayta, or placebo. The primary outcome is cognition as assessed by the Alzheimer’s Disease Clinical Study Activities of Daily Living (ADCS-ADL), but the trial will also use the investigational PET tracer UCB-J, which binds to the synaptic vesicle glycoprotein 2A, in an attempt to monitor synapse density before and after treatment (see company press release; Jul 2016 news).
In summer 2018, a Phase 1b target engagement study at the University of Pennsylvania will start enrolling 18 people whose mild to moderate AD is confirmed by amyloid PET. Called COG0104 or SNAP, it will compare single injections of 90, 280, or 560 mg of Elayta or placebo for their ability to displace Aβ oligomers and clear them into the CSF, as measured by a CSF Aβ oligomer assay.
Also in summer 2018, a Phase 2 multi-center study is expected to begin enrolling 24 people with mild to moderate AD as confirmed by amyloid PET for a six-month course of 100 or 300 mg of Elayta, or placebo. As of May 22, 2018, this trial lists CT1812 pharmacodynamic effects on CSF biomarkers, specifically as assessed by CSF neurogranin levels, as primary outcome.
For all trials of this compound, see clinicaltrials.gov.
PATENT
WO 2015116923
https://patents.google.com/patent/WO2015116923A1
There are only five medications currently FDA-approved for the treatment of Alzheimer’s Disease (AD). Four are cholinesterase inhibitors: tacrine (COGNEX®; Sciele), donepezil (ARICEPT®; Pfizer), rivastigmine (EXELON®; Novartis), and galantamine (RAZADYNE®; Ortho-McNeil-Janssen). Donepezil, rivastigmine, and galantamine are successors to tacrine, a first generation compound rarely prescribed because of the potential for hepatotoxicity; they are roughly equally efficacious at providing symptomatic improvement of cognition and function at all stages of AD. The fifth approved medication is memantine (NAMENDA®; Forest), a low-affinity, use dependent N-methyl-D-aspartate glutamate receptor antagonist that offers similar benefits, but only in moderate to severe AD. The clinical effects of these compounds are small and impermanent, and currently available data are inconclusive to support their use as disease modifying agents. See, e.g., Kerchner et al, 2010, Bapineuzumab, Expert Opin Biol Ther., 10(7): 1121-1130. Clearly, alternative approaches to treatment of AD are required.
[004] Certain isoindoline compounds are provided that act as sigma-2 receptor functional antagonists and inhibit the deleterious effects of soluble Αβ oligomers. In some embodiments, isoindoline sigma-2 receptor antagonist compounds and compositions are used to treat or prevent synaptic dysfunction in a subject.
Example 21 illustrates representative preparation of 2-(Tert-butoxy)-
4-(3-methyl-3-(5-(methylsulfonyl)isoindolin-2-yl)butyl)phenol, Example Compound 62, as shown in Scheme 17.
10 Compound 62
[0534] Scheme 17: Procedure for preparation of 2-(Tert-butoxy)-4-(3- methyl-3-(5-(methylsulfonyl)isoindolin-2-yl)butyl)phenol, Example Compound 62.
[0535] Preparation of compound l(Scheme 17): To a glass pressure -bottle at -30 °C containing a mixture of catechol (50.0 g, 454 mmol, 1.0 eq), concentrated sulfuric acid (0.3 mL) in dichloromethane (200 mL), isobutene (152.6 g, 2.72 mol, 6.0 eq) was condensed. After sealing the pressure-bottle with a threaded Teflon cap tipped with a Teflon-protected rubber O-ring, the mixture was heated at 35 °C for 3 h until a clear solution was obtained. After cooling (-30 °C), triethylamine (1.5 mL, 10.8 mmol) was added and the mixture was concentrated. The residue was suspended in 0.5 M NaOH (1 L) and stirred for 10 min. The dark-green colored solution was washed with petroleum ether (2x 100 mL) and the washing layers were reextracted with 0.5 M NaOH (3x 100 mL). The combined aqueous layers were brought to pH 7-8 with 2 N HCl (400 mL), and extracted with ethyl acetate (2* 1 L), dried over sodium sulfate and concentrated to afford product 1 (67.7 g, 90%) as a colorless oil, which was used directly for the next step reaction without further purification. TLC: PE/EA = 50/1 ; Rf (Catechol) = 0.1 ; Rf (Compound 1) = 0.6.
[0536] Preparation of compound 2 (Scheme 17): To a stirred solution of compound 1 (1 12.2 g, 676 mmol, 1.2 eq) and potassium iodide (1 12.2 g, 676 mmol, 1.0 eq) in methanol (2 L) at 0 °C was slowly added sodium hydroxide (27.0 g, 676 mmol, 1.0 eq), followed with aqueous sodium chlorite (7% aq., 718.8 mL, 710 mmol, 1.05 eq) dropwise over 3 h while keeping the reaction below 0 °C. The mixture was stirred at 0 °C for another 30 min and neutralized by adding 2 N HCl at 0 °C till pH 7, extracted with DCM (2 x 1 L). The organic layers were dried over sodium sulfate and concentrated to afford product 2 (179.8 g, 91%). TLC: PE/EA = 50/1; Rf(Compound 1) = 0.6 ; Rf (Compound 2) = 0.6.
[0537] Preparation of compound 3(Scheme 17): To a stirred solution of compound 2 (179.8 g, 616 mmol, 1.0 eq) and triethylamine (186.6 g, 1.85 mol, 3.0 eq) in dichloromethane (2 L) at 0 °C was slowly added acetyl chloride (53.2 g, 677 mmol, 1.1 eq). The mixture was stirred at 0 °C for another 30 min, and warmed up to rt, and stirred at rt for 3 h, water (1 L) was added into the reaction mixture and the organic layer was washed with brine, dried over sodium sulfate and concentrated to afford product 3 (206 g, 100%), which was used directly to the next step without further purification. TLC: PE/EA = 50/1; Rf (Compound 2) = 0.6; Rf (Compound 3) = 0.5.
[0538] Preparation of compound 4 (Scheme 17): To a stirred solution of compound 3 (206 g, 616 mmol, 1.0 eq) in triethylamine (4.0 L) was added 2- methylbut-3-yn-2-amine (102.5 g, 1.23 mol, 2.0 eq), Pd(PPh3)2Cl2 (15.1 g, 18.5 mmol, 0.03 eq) and copper(I) iodide (5.9 g, 31 mmol, 0.05 eq) and resulting mixture was stirred at rt for 17 h. The solvent was removed under reduced pressure and the crude product was purified by silica gel chromatography to afford the title compound 4 (132.7 g, 74%). TLC: PE/EA = 1/1; Rf (Compound 3) = 0.9; Rf (Compound 4) = 0.3. [0539] Preparation of compound 5(Scheme 17): To a stirred solution of compound 4 (104.5 g, 0.36 mol) in ethanol (1.5 L) was added Pd/C (10% wt, 10.5 g). The mixture was stirred under hydrogen (balloon) overnight, and filtered. The filtrate was evaporated to dryness to afford compound 5 (106.3 g, 100%), which was used directly to the next step without further purification. TLC: PE/EA = 1/1; Rf(Compound 4) = 0.3 ; Rf (Compound 5) = 0.3.
[0540] Preparation of compound 6 (Scheme 17): To a solution of o-xylene
(115.7 g, 1.09 mol, 1.0 eq) in chloroform (1.0 L) at 0 °C was added C1S03H (254 g, 2.18 mol, 2.0 eq) dropwise. After the addition, the reaction mixture was stirred at room temperature for 2 days, and poured into ice. The crude mixture was extracted with dichloromethane (3 x 1.0 L). The organic layers were combined, dried over anhydrous sodium sulfate, concentrated to afford the crude compound 6 (161.5 g, 80%) as a white solid, which was used directly to the next step without further purification. TLC: PE/EA = 5/1; Rf (Compound 6) = 0.7.
[0541] General procedure for the preparation of compound 7 (Scheme
17): To a stirred solution of compound 6 (161.5 g, 0.87 mol, 1.0 eq) in saturated sodium sulfite solution (273 g, 2.17 mol, 2.5 eq, in 2.0 L of water) was added dropwise 32% NaOH (69.4 g, 1.73 mol, 2.0 eq) till the solution reached pH 9. After stirring at rt overnight, the reaction mixture was acidified with cone. HC1 in ice- cooling bath till pH 1. The precipitate was filtered, and washed with ice-water (2x), dried in vacuo to afford the crude product 7 (131 g, 88%), which was used directly for next step without further purification. TLC: PE/EA = 5/1; Rf (Compound 6) = 0.7; Rf (Compound 7) = 0.6.
[0542] Preparation of compound 8 (Scheme 17): To a stirred solution of compound 7 (130 g, 0.76 mol, 1.0 eq) and potassium carbonate (211 g, 1.53 mol, 2.0 eq) in DMF (300 mL) was added iodomethane (96 mL, 1.53 mol, 2.0 eq). The reaction was stirred at 40 °C overnight. The reaction mixture was evaporated to dryness, extracted with ethyl acetate. The organic layers were washed with water and brine, dried over sodium sulfate and concentrated, purified by flash column chromatography (PE: EA,10: 1 ~ 5: 1) to afford compound 8 (85.2 g, 61%). TLC: PE/EA = 5/1; Rf (Compound 7) = 0.6; Rf (Compound 8) = 0.3. [0543] Preparation of compound 9 (Scheme 17):To a stirred solution of compound 8 (78.2 g, 424 mmol, 1.0 eq) in 1 ,2-dichloroethane (1.2 L), were added N-bromosuccinimide (166 g, 934 mmol, 2.2 eq) and AIBN (6.9 g, 42.4 mmol, 0.1 eq). The reaction was stirred at reflux overnight. The reaction was diluted with water and dichloromethane. The organic layer was collected, and dried over sodium sulfate and concentrated, purified by flash column chromatography to afford compound 9, which was further recrystallized from hot methanol to afford the pure product 8 (75 g, 52%). TLC: PE/EA = 5/1; Rf (Compound 8) = 0.3; Rf (Compound 9) = 0.2.
[0544] Preparation of compound 10 (Scheme 17):To a stirred solution of compound 5 (46 g, 157 mmol, 1.0 eq) and compound 9 (53.5 g, 157 mmol, 1.0 eq) in THF (460 mL) was added triethylamine (47.7 g, 472 mmol, 3.0 eq). The reaction was stirred at 40 °C overnight, filtered and the filtrate was evaporated to dryness and purified by flash column chromatography to afford compound 10 (45 g, 63%). TLC: PE/EA = 1/1; Rf (Compound 5) = 0.3; Rf (Compound 9) = 1.0; Rf (Compound 10) = 0.4.
[0545] Preparation of Compound 62 (Scheme 17):To a stirred solution of compound 10 (45 g, 98.4 mmol) in methanol (300 mL) was added sodium methoxide (844 mg, 15.6 mmol, 0.16 eq) in one portion. The solution was stirred at rt overnight. Water (250 mL) was added dropwise into the reaction mixture over 1 h, the mixture was stirred at rt for 2 h, and filtered. The white solid was collected and dried on vacuum overnight to afford pure example Compound 62 base (38 g, 89%>). TLC: PE/EA = 1/1; Rf (Compound 10) = 0.4; Rf (Compound 62) = 0.4; ESI-MS: 432 (M+l)+; 1H NMR (400 MHz, CDC13) δ 7.80-7.78 (m, 2H). 7.40-7.38 (m, 1H), 6.87-6.79 (m, 3H), 5.58 (s, 1H), 4.11 (s, 4H), 3.05 (s, 3H), 2.61-2.57 (m, 2H), 1.76- 1.72 (m, 2H), 1.48 (s, 9H), 1.18 (s, 6H). Example 22: Preparation of (2-(4-(4-Hydroxy-3-methoxyphenyl)-2- methylbutan-2-yl)isoindolin-4-yl)(piperazin-l-yl)methanone,
REFERENCES
1: Grundman M, Morgan R, Lickliter JD, Schneider LS, DeKosky S, Izzo NJ,
Guttendorf R, Higgin M, Pribyl J, Mozzoni K, Safferstein H, Catalano SM. A phase
1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a
novel therapeutic candidate for Alzheimer’s disease. Alzheimers Dement (N Y).
2019 Jan 23;5:20-26. doi: 10.1016/j.trci.2018.11.001. eCollection 2019. PubMed
PMID: 30723776; PubMed Central PMCID: PMC6352291.
Paper Citations
- Catalano S, Grundman M, Schneider LS, DeKosky S, Morgan R, Higgin M, Pribyl J, Mozzoni K, Izzo NJ, Safferstein H, Lickliter J. A Two-Part, Double-Blind, Placebo-Controlled, Phase 1 Study of the Safety and Pharmacokinetics of Single and Multiple Ascending Doses of Ct1812 in Healthy Volunteers. Alzheimer’s & Dementia, July 2016, Volume 12, Issue 7, Supplement
- Catalano S, Grundman M, Schneider LS, DeKosky S, Morgan R, Guttendorf R, Higgin M, Pribyl J, Mozzoni K, Izzo NJ, Safferstein H. A Phase 1 Safety Trial of the aβ Oligomer Receptor Antagonist CT1812. Alzheimer’s & Dementia, July 2017, Volume 13, Issue 7
- Izzo NJ, Staniszewski A, To L, Fa M, Teich AF, Saeed F, Wostein H, Walko T 3rd, Vaswani A, Wardius M, Syed Z, Ravenscroft J, Mozzoni K, Silky C, Rehak C, Yurko R, Finn P, Look G, Rishton G, Safferstein H, Miller M, Johanson C, Stopa E, Windisch M, Hutter-Paier B, Shamloo M, Arancio O, LeVine H 3rd, Catalano SM. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS One. 2014;9(11):e111898. Epub 2014 Nov 12 PubMed.
- Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, Rehak C, Yurko R, Look G, Rishton G, Safferstein H, Cruchaga C, Goate A, Cahill MA, Arancio O, Mach RH, Craven R, Head E, LeVine H 3rd, Spires-Jones TL, Catalano SM. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One. 2014;9(11):e111899. Epub 2014 Nov 12PubMed.
/////CT-1812, CT 1812, CT1812, Alzheimers , Cognition Therapeutics, Elayta, phase 2, Cognition disorders
OC1=CC=C(CCC(C)(N2CC3=C(C=C(S(=O)(C)=O)C=C3)C2)C)C=C1OC(C)(C)C
















