GIMERACIL
C5H4ClNO2, 145.54
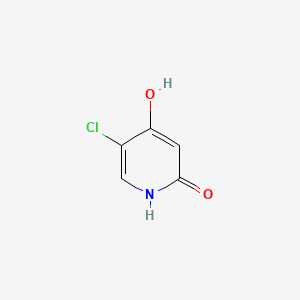
5-chloro-4-hydroxy-1H-pyridin-2-one
5-Chloro-2,4-dihydroxypyridine
5-Chloro-4-hydroxy-2(1H)-pyridone
CCDSCO APPROVED,01.02.2022

Gimeracil bulk & Oteracil potassium bulk and Tegafur 15mg/20mg, Gimeracil 4.35mg/5.8mg and Oteracil 11.8mg/15.8mg capsules
indicated in adults for the treatment of advanced gastric cancer when given in combination with cisplatin.
| Combination of | |
|---|---|
| Tegafur | Antineoplastic drug |
| Gimeracil | Enzyme inhibitor |
| Oteracil | Enzyme inhibitor |
| Clinical data | |
| Trade names | Teysuno, TS-1 |
| Other names | S-1[1] |
| AHFS/Drugs.com | UK Drug Information |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
Tegafur/gimeracil/oteracil, sold under the brand names Teysuno and TS-1,[3][4] is a fixed-dose combination medication used for the treatment of advanced gastric cancer when used in combination with cisplatin,[3] and also for the treatment of head and neck cancer, colorectal cancer, non–small-cell lung, breast, pancreatic, and biliary tract cancers.[5]: 213
The most common severe side effects when used in combination with cisplatin include neutropenia (low levels of neutrophils, a type of white blood cell), anaemia (low red blood cell counts) and fatigue (tiredness).[3]
Tegafur/gimeracil/oteracil (Teysuno) was approved for medical use in the European Union in March 2011.[3] It has not been approved by the U.S. Food and Drug Administration (FDA).[5]: 213
Medical uses
In the European Union tegafur/gimeracil/oteracil is indicated in adults for the treatment of advanced gastric cancer when given in combination with cisplatin.[3]
Contraindications
In the European Union, tegafur/gimeracil/oteracil must not be used in the following groups:
- people receiving another fluoropyrimidine (a group of anticancer medicines that includes tegafur/gimeracil/oteracil) or who have had severe and unexpected reactions to fluoropyrimidine therapy;[3]
- people known to have no DPD enzyme activity, as well as people who, within the previous four weeks, have been treated with a medicine that blocks this enzyme;[3]
- pregnant or breastfeeding women;[3]
- people with severe leucopenia, neutropenia, or thrombocytopenia (low levels of white cells or platelets in the blood);[3]
- people with severe kidney problems requiring dialysis;[3]
- people who should not be receiving cisplatin.[3]
Mechanism of action
Tegafur is the actual chemotherapeutic agent. It is a prodrug of the active substance fluorouracil (5-FU).[3] Tegafur, is a cytotoxic medicine (a medicine that kills rapidly dividing cells, such as cancer cells) that belongs to the ‘anti-metabolites’ group. Tegafur is converted to the medicine fluorouracil in the body, but more is converted in tumor cells than in normal tissues.[3] Fluorouracil is very similar to pyrimidine.[3] Pyrimidine is part of the genetic material of cells (DNA and RNA).[3] In the body, fluorouracil takes the place of pyrimidine and interferes with the enzymes involved in making new DNA.[3] As a result, it prevents the growth of tumor cells and eventually kills them.[3]
Gimeracil inhibits the degradation of fluorouracil by reversibly blocking the dehydrogenase enzyme dihydropyrimidine dehydrogenase (DPD). This results in higher 5-FU levels and a prolonged half-life of the substance.[6]
Oteracil mainly stays in the gut because of its low permeability, where it reduces the production of 5-FU by blocking the enzyme orotate phosphoribosyltransferase. Lower 5-FU levels in the gut result in a lower gastrointestinal toxicity.[6]
Within the medication, the molar ratio of the three components (tegafur:gimeracil:oteracil) is 1:1:0.4.[7]
The maximum tolerated dose differed between Asian and Caucasian populations (80 mg/m2 and 25 mg/m2 respectively), perhaps due to differences in CYP2A6 genotype.[5]: 213
Research
It is being developed for the treatment of hepatocellular carcinoma.[8] and has activity in esophageal,(Perry Chapter 33) breast,[citation needed] cervical,[citation needed] and colorectal cancer.[9]
References
- ^ Liu TW, Chen LT (201). “S-1 with leucovorin for gastric cancer: how far can it go?”. Lancet Oncol. 17 (1): 12–4. doi:10.1016/S1470-2045(15)00478-7. PMID 26640038.
- ^ “Teysuno 20mg/5.8mg/15.8mg hard capsules – Summary of Product Characteristics (SmPC)”. (emc). Retrieved 30 July 2020.
- ^ Jump up to:a b c d e f g h i j k l m n o p q r “Teysuno EPAR”. European Medicines Agency (EMA). Retrieved 30 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ “ティーエスワン 患者さん・ご家族向け総合情報サイト | 大鵬薬品工業株式会社”.
- ^ Jump up to:a b c DeVita, DeVita; Lawrence, TS; Rosenberg, SA (2015). DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology (10th ed.). LWW. ISBN 978-1451192940.
- ^ Jump up to:a b A. Klement (22 July 2013). “Dreier-Kombination gegen Magenkrebs: Teysuno”. Österreichische Apothekerzeitung (in German) (15/2013): 23.
- ^ Peters GJ, Noordhuis P, Van Kuilenburg AB et al. (2003). “Pharmacokinetics of S-1, an oral formulation of ftorafur, oxonic acid and 5-chloro-2,4-dihydroxypyridine (molar ratio 1:0.4:1) in patients with solid tumors”. Cancer Chemother. Pharmacol. 52 (1): 1–12. doi:10.1007/s00280-003-0617-9. PMID 12739060. S2CID 10858817.
- ^ “BCIQ”.
- ^ Miyamoto Y, Sakamoto Y, Yoshida N, Baba H (2014). “Efficacy of S-1 in colorectal cancer”. Expert Opin Pharmacother. 15 (12): 1761–70. doi:10.1517/14656566.2014.937706. PMID 25032886. S2CID 23637808.
External links
- “Tegafur”. Drug Information Portal. U.S. National Library of Medicine.
- “Gimeracil”. Drug Information Portal. U.S. National Library of Medicine.
- “Oteracil”. Drug Information Portal. U.S. National Library of Medicine.
Gimeracil is an adjunct to antineoplastic therapy, used to increase the concentration and effect of the main active componets within chemotherapy regimens. Approved by the European Medicines Agency (EMA) in March 2011, Gimeracil is available in combination with Oteracil and Tegafur within the commercially available product “Teysuno”. The main active ingredient in Teysuno is Tegafur, a pro-drug of Fluorouracil (5-FU), which is a cytotoxic anti-metabolite drug that acts on rapidly dividing cancer cells. By mimicking a class of compounds called “pyrimidines” that are essential components of RNA and DNA, 5-FU is able to insert itself into strands of DNA and RNA, thereby halting the replication process necessary for continued cancer growth.
Gimeracil’s main role within Teysuno is to prevent the breakdown of Fluorouracil (5-FU), which helps to maintin high enough concentrations for sustained effect against cancer cells 2. It functions by reversibly and selectively blocking the enzyme dihydropyrimidine dehydrogenase (DPD), which is involved in the degradation of 5-FU 1. This allows higher concentrations of 5-FU to be achieved with a lower dose of tegafur, thereby also reducing toxic side effects.
/////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////


SYNTHESIS

SYN
https://europepmc.org/article/pmc/pmc7717319
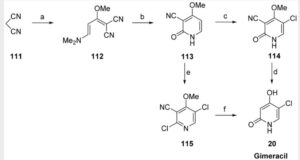
Synthesis of Gimeracil 20a
aReagents and conditions: (a) CH3C(OCH3)3, MeOH, then (CH3)2NHCH(OCH3)2, reflux, 92%; (b) aq AcOH, 130 °C, 2 h, 95%; (c) SO2Cl2, HOAc, 50 °C, 0.5 h, 91%; (d) 40% H2SO4, 130 °C, 4 h, 91%; (e) SO2Cl2, HOAc, 50 °C, 45 min, 86%; (f) 75% H2 SO4, 140 °C, 3 h, then NaOH, then pH 4–4.5, 89%

In 1953, Kolder and Hertog reported a synthesis of the TS-1 additive gimeracil 20, which was completed in seven steps using 4-nitropyridine N-oxide as starting material.222 Later, Yano et al. reported an alternative gram-scale synthesis (Scheme 15).223 The one-pot, three component condensation of malononitrile 111, 1,1,1-trimethoxyethane, and 1,1-dimethyoxytrimethylamine generated the dicyano intermediate 112, which was into 2(1H)-pyridinone 113.224 Selective chlorination of 113 was followed by acid-mediated demethylation, hydrolysis, and decarboxylation, to afford gimeracil 20. Interestingly, Xu et al. found that treatment of intermediate 113 with sulfuryl chloride resulted in dichloro 115 formation, which could still be converted to gimeracil 20 by treatment with sulfuric acid.225
//////////GIMERACIL, APPROVALS 2022, INDIA 2022
OC1=CC(=O)NC=C1Cl