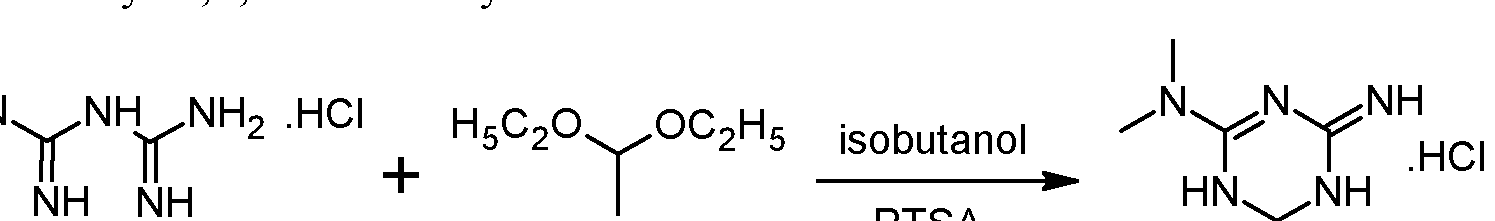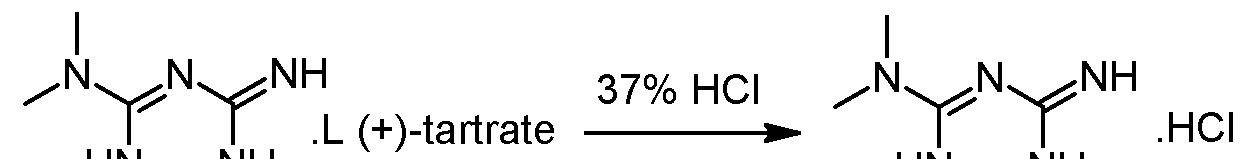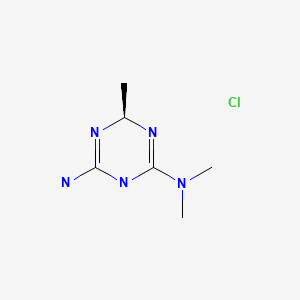

Meglimin hydrochloride
Twymeeg
| Formula |
C6H13N5. HCl
|
| CAS |
775351-61-6 (HCl). , C6H14ClN5 191.66
CAS 775351-65-0, FREEFORM 155.20 |
| Mol weight |
191.6619
|
AntidiabeticAPPROVED PMDA JAPAN2021/6/23, イメグリミン塩酸塩
(4R)-6-N,6-N,4-trimethyl-1,4-dihydro-1,3,5-triazine-2,6-diamine
DB12509
NCGC00378621-02
HY-14771
Q6003719
UNII-UU226QGU97
UU226QGU97
1,3,5-Triazine-2,4-diamine,1,6-dihydro-N,N,6-trimethyl-,(+)-(9CI)
(4R)-6-N,6-N,4-trimethyl-1,4-dihydro-1,3,5-triazine-2,6-diamine
Imeglimin [INN]
Emd 387008 (R-imeglimin) HCl
EMD-387008
Imeglimin is an experimental drug being developed as an oral anti-diabetic.[1][2] It is an oxidative phosphorylation blocker that acts to inhibit hepatic gluconeogenesis, increase muscle glucose uptake, and restore normal insulin secretion. It will be the first of a new class of anti-diabetic if it is approved.


PATENT
https://patents.google.com/patent/WO2012072663A1/en
EXAMPLES
Example 1 : Synthesis and isolation of (+)-2-amino-3,6-dihydro-4-dimethylamino-6- methyl-l,3,5-triazine hydrochloride by the process according to the invention
Preliminary step: Synthesis of racemic 2-amino-3,6-dihydro-4-dimethylamino- 6-methyl-l,3,5-triazine hydrochloride:
Metformin hydrochloride is suspended in 4 volumes of isobutanol. Acetaldehyde diethylacetal (1.2 eq.) and para-toluenesulfonic acid (PTSA) (0.05 eq) are added and the resulting suspension is heated to reflux until a clear solution is obtained. Then 2 volumes of the solvent are removed via distillation and the resulting suspension is cooled to 20°C. The formed crystals are isolated on a filter dryer and washed with isobutanol (0.55 volumes). Drying is not necessary and the wet product can be directly used for the next step.
Acetaldehyde diethylacetal can be replaced with 2,4,6-trimethyl-l,3,5-trioxane (paraldehyde).
– Steps 1 and 2: formation of the diastereoisomeric salt and isolation of the desired diastereoisomer
Racemic 2-amino-3,6-dihydro-4-dimethylamino-6-methyl-l,3,5-triazine hydrochloride wet with isobutanol (obtained as crude product from preliminary step without drying) and L-(+)-Tartaric acid (1 eq.) are dissolved in 2.2 volumes of methanol at 20-40°C. The obtained clear solution is filtered and then 1 equivalent of triethylamine (TEA) is added while keeping the temperature below 30°C. The suspension is heated to reflux, stirred at that temperature for 10 minutes and then cooled down to 55°C. The temperature is maintained at 55°C for 2 hours and the suspension is then cooled to 5- 10°C. After additional stirring for 2 hours at 5-10°C the white crystals are isolated on a filter dryer, washed with methanol (2 x 0.5 Vol) and dried under vacuum at 50°C. The yield after drying is typically in the range of 40-45%
– Steps 3 and 4: transformation of the isolated diastereoisomer of the tartrate salt into the hydrochloride salt and recovery of the salt
γ ethanol HN^NH
(+) 2-amino-3,6-dihydro-4-dimethylamino-6-methyl-l,3,5-triazine tartrate salt is suspended in 2 volumes of ethanol and 1.02 equivalents of HCl-gas are added under vacuum (-500 mbar). The suspension is heated to reflux under atmospheric pressure (N2) and 5% of the solvent is removed via distillation. Subsequent filtration of the clear colourless solution into a second reactor is followed by a cooling crystallization, the temperature is lowered to 2°C. The obtained suspension is stirred at 2°C for 3 hours and afterwards the white crystals are isolated with a horizontal centrifuge. The crystal cake is washed with ethanol and dried under vacuum at 40°C. The typical yield is 50-55% and the mother liquors can be used for the recovery of about 25-30%) of (+)-2-amino- 3,6-dihydro-4-dimethylamino-6-methyl-l,3,5-triazine tartrate.
Example 2: Modification of the solvent of steps 3 and 4
– Steps 3 and 4: transformation of the isolated diastereoisomer of the tartrate salt into the hydrochloride salt and recovery of the salt
HN^NH acetone HN^NH
(+) 2-amino-3,6-dihydro-4-dimethylamino-6-methyl-l,3,5-triazine tartrate salt synthesized according to steps 1 and 2 of example 1 is suspended in 1 volume (based on total amount of (+) 2-amino-3,6-dihydro-4-dimethylamino-6-methyl-l,3,5-triazine tartrate salt) of acetone at 20°C. To this suspension 1.01 equivalents of 37% Hydrochloric acid are added. The suspension is heated to reflux under atmospheric pressure (N2) and water is added until a clear solution is obtained. 1.5 vol of acetone are added at reflux temperature. The compound starts crystallising and the obtained suspension is kept at reflux for 2 hours followed by a cooling crystallization to 0°C. The obtained suspension is stirred at 0°C for 2 hours and the white crystals are isolated by centrifugation. The crystal cake is washed with isopropanol and dried under vacuum at 40°C in a continuous drying oven.
References
- ^ Vuylsteke, V; Chastain, L. M; Maggu, G. A; Brown, C (2015). “Imeglimin: A Potential New Multi-Target Drug for Type 2 Diabetes”. Drugs in R&D. 15 (3): 227–232. doi:10.1007/s40268-015-0099-3. PMC 4561051. PMID 26254210.
- ^ Dubourg, J; Fouqueray, P; Thang, C; Grouin, JM; Ueki, K (April 2021). “Efficacy and Safety of Imeglimin Monotherapy Versus Placebo in Japanese Patients With Type 2 Diabetes (TIMES 1): A Double-Blind, Randomized, Placebo-Controlled, Parallel-Group, Multicenter Phase 3 Trial”. Diabetes Care. 44 (4): 952–959. doi:10.2337/dc20-0763. PMID 33574125.
/////////Imeglimin hydrochloride, Twymeeg, JAPAN 2021, APPROVALS 2021, Antidiabetic, イメグリミン塩酸塩, ATI DIABETES, DIABETES, Imeglimin
CC1N=C(NC(=N1)N(C)C)N.Cl