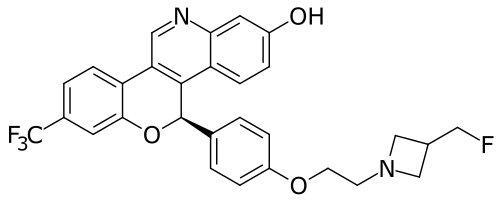

Imlunestrant
CAS 2408840-26-4
as tosylate: 2408840-41-3
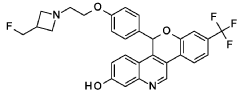
(5R)-5-[4-[2-[3-(fluoromethyl)azetidin-1-yl]ethoxy]phenyl]-8-(trifluoromethyl)-5H-chromeno[4,3-c]quinolin-2-ol
- (5r)-5-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)phenyl)-8-(trifluoromethyl)-5h-(1)benzopyrano(4,3-c)quinolin-2-ol
- 5h-(1)benzopyrano(4,3-c)quinolin-2-ol, 5-(4-(2-(3-(fluoromethyl)-1-azetidinyl)ethoxy)phenyl)-8-(trifluoromethyl)-, (5r)-
MF C29H24F4N2O3 MW 524.516
FDA 9/25/2025, Inluriyo, LY3484356, LY-3484356, To treat estrogen receptor-positive, human epidermal growth factor receptor 2-negative, estrogen receptor-1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy
Imlunestrant, sold under the brand name Inluriyo, is an anti-cancer medication used for the treatment of breast cancer.[1] It is an is an estrogen receptor antagonist.[1] It is used as the salt, imlunestrant tosylate.[2] It is taken by mouth.[1] It was developed by Eli Lilly and Company.[2]
The most common adverse events and laboratory abnormalities include decreased hemoglobin, musculoskeletal pain, decreased calcium, decreased neutrophils, increased AST, fatigue, diarrhea, increased ALT, increased triglycerides, nausea, decreased platelets, constipation, increased cholesterol, and abdominal pain.[2]
Imlunestrant was approved for medical use in the United States in September 2025.[2]
SYN
- Imlunestrant with or without Abemaciclib in Advanced Breast CancerPublication Name: The New England journal of medicinePublication Date: 2025-03-27PMID: 39660834DOI: 10.1056/nejmoa2410858
- Targeting the Estrogen Receptor for the Treatment of Breast Cancer: Recent Advances and ChallengesPublication Name: Journal of Medicinal ChemistryPublication Date: 2023-06-28PMID: 37377342DOI: 10.1021/acs.jmedchem.3c00136
- Novel endocrine therapies: What is next in estrogen receptor positive, HER2 negative breast cancer?Publication Name: Cancer Treatment ReviewsPublication Date: 2023-06PMID: 37146385DOI: 10.1016/j.ctrv.2023.102569
- Oral Selective Estrogen Receptor Degraders (SERDs) as a Novel Breast Cancer Therapy: Present and Future from a Clinical PerspectivePublication Name: International Journal of Molecular SciencesPublication Date: 2021-07-22PMCID: PMC8345926PMID: 34360578DOI: 10.3390/ijms22157812
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US281655517&_cid=P12-MG7DCV-14904-1
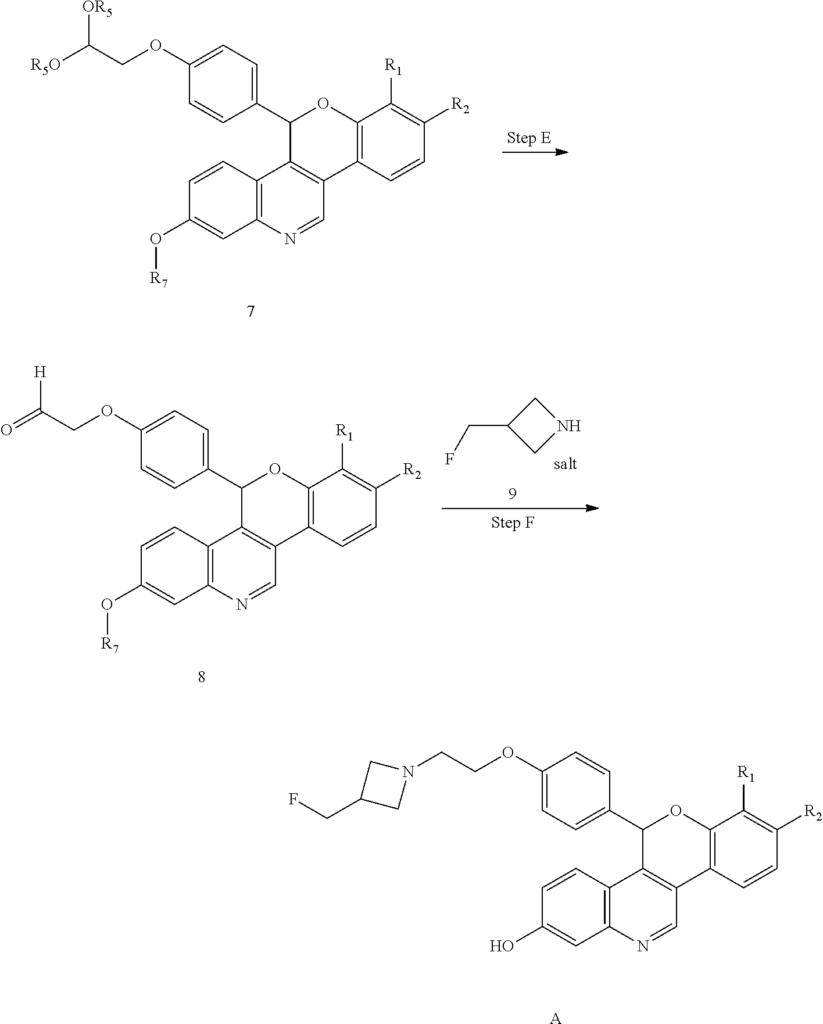
Example 1A
5-(4-{2-[3-(Fluoromethyl)azetidin-1-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H-[1]benzopyrano[4,3-c]quinolin-2-ol, Isomer 1Separate the two enantiomers of 5-(4-{2-[3-(fluoromethyl)azetidin-1-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H-[1]benzopyrano[4,3-c]quinolin-2-ol by chiral SFC with the following conditions: Column: LUX® Cellulose-1, 5×25 cm; eluting with a mobile phase of 30% iPrOH (with 0.5% DMEA) in CO 2; column temperature: 40° C.; flow rate: 300 g/minute; UV detection wavelength: 270 nm to give Example 1A as the first eluting enantiomer (Isomer 1). ES/MS (m/z): 525.2 (M+H). Confirm enantiomeric enrichment of Isomer 1 by chiral analytical SFC, >99% ee, t (R): 1.30 minutes; column: CHIRALCEL® OD-H, 4.6×150 mm; eluting with a mobile phase of 30% MeOH (0.2% IPA) in CO 2; column temperature: 40° C.; flow rate: 5 mL/minute; UV detection wavelength: 225 nm. Isolate the title compound of Example 1B to give the second eluting enantiomer (Isomer 2). ES/MS (m/z): 525.2 (M+H). Confirm enantiomeric enrichment of Isomer 2 by chiral analytical SFC, 98% ee, t (R): 2.03 minutes; column: CHIRALCEL® OD-H, 4.6×150 mm; eluting with a mobile phase of 30% MeOH (0.2% IPA) in CO 2; column temperature: 40° C.; flow rate: 5 mL/minute; UV detection wavelength: 225 nm.
Alternate Preparation Example 1B
Crystalline 5-(4-{2-[3-(Fluoromethyl)azetidin-1-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H-[1]benzopyrano[4,3-c]quinolin-2-ol, Isomer 2
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020014435&_cid=P12-MG7DHN-18354-1
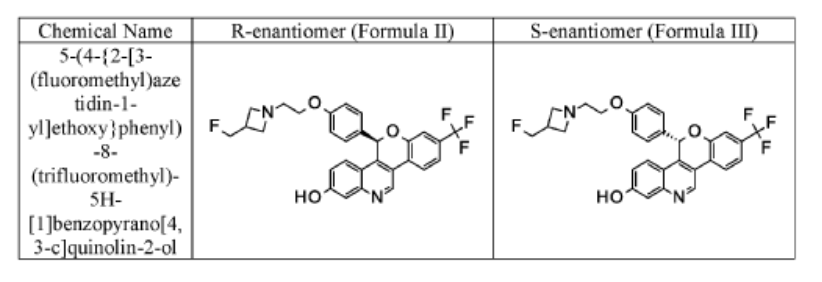
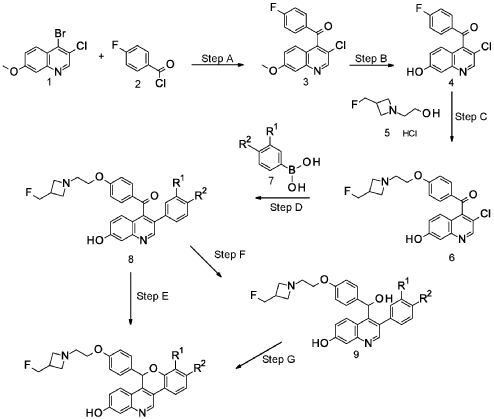
EXAMPLE 1
Racemic 5-(4-{2-[3-(Fluoromethyl)azetidin-l-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H- [ 1 ]benzopyrano[4,3 -c]quinolin-2-ol
Cool a solution of (4-{2-[3-(fluoromethyl)azetidin-l-yl]ethoxy}phenyl){3-[2-fluoro-4-(trifluoromethyl)phenyl]-7-hydroxyquinolin-4-yl}methanone (5.27 g, 9.71 mmol) in 1,4-dioxane (100 mL) to 5 °C. Add lithium triethylborohydride (1 M in THF, 30.0 mL, 30.0 mmol). Remove the cooling bath and stir for 1.5 hours at room temperature. Quench the mixture with water. Add saturated NH4Cl solution and EtOAc. Separate the layers and extract the aqueous layer with EtOAc. Combine the organic extracts, dry over anhydrous MgS04, filter, and concentrate the filtrate. Dissolve the crude residue in THF (100 mL).
Add sodium hydride (60% in mineral oil, 1.94 g, 48.5 mmol). Reflux the solution for 1.5 hours. Add additional sodium hydride (60% in mineral oil, 1.94 g, 48.5 mmol), then reflux for an additional 30 minutes. Cool the solution to room temperature and quench with water. Add EtOAc and saturated NH4Cl solution. Separate the layers and extract the aqueous layer with EtOAc. Combine the organic extract, dry over anhydrous MgS04, filter, and concentrate the filtrate. Purify the residue by silica gel column chromatography eluting with a gradient of 5-7% MeOH in DCM to give the title compound (3.70 g, 72%) as a light yellow foam. ES/MS (m/z): 525.2 (M+H).
Prepare the following compounds in a manner essentially analogous to the method of Example 1, with the following variations in procedure. For the reduction, use 3 to 5 equivalents of lithium triethylborohydride with reaction times from 30 minutes to one hour and drying of the organic layers over magnesium sulfate or sodium sulfate. ETse the crude residue directly or purify by silica gel column chromatography eluting with a gradient of 0-5-7.5-10% MeOH in DCM before cyclization. Complete the cyclization by refluxing in THF for up to 16 hours, or in DMF, from 2 hours at room temperature for Ex 2, to 2 hours at 85 °C for Ex 8. Extract with DCM or EtOAc and dry organic layers over magnesium sulfate or sodium sulfate. Purify by silica gel column chromatography using up to 10% (MeOH or 7 M ammoniated MeOH) in DCM (Ex 2: gradient 0-10% MeOH in DCM; Ex 5: gradient 4-10% 7 M ammoniated MeOH in DCM; Ex 8: gradient 5-7.5% 7 M ammoniated MeOH in DCM) or by high pH reversed phase HPLC as noted.
EXAMPLE 1A
-(4-{2-[3-(Fluoromethyl)azetidin-l-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H- [l]benzopyrano[4,3-c]quinolin-2-ol, Isomer 1
and
EXAMPLE 1B
5-(4-{2-[3-(Fluoromethyl)azetidin-l-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H- [l]benzopyrano[4,3-c]quinolin-2-ol, Isomer 2
Separate the two enantiomers of 5-(4-{2-[3-(fluoromethyl)azetidin-l-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H-[l]benzopyrano[4,3-c]quinolin-2-ol by chiral SFC with the following conditions: Column: LUX® Cellulose-l, 5 x 25 cm; eluting with a mobile phase of 30% iPrOH (with 0.5% DMEA) in C02; column temperature: 40 °C; flow rate: 300 g/minute; UV detection wavelength: 270 nm to give Example 1 A as the first eluting enantiomer (Isomer 1). ES/MS (m/z): 525.2 (M+H). Confirm enantiomeric enrichment of Isomer 1 by chiral analytical SFC, >99% ee, /(R>: 1.30 minutes; column: CHFRALCEL® OD-H, 4.6 x 150 mm; eluting with a mobile phase of 30% MeOH (0.2% IP A) in C02; column temperature: 40 °C; flow rate: 5 mL/minute; UV detection wavelength: 225 nm. Isolate the title compound of Example 1B to give the second eluting enantiomer (Isomer 2). ES/MS (m/z): 525.2 (M+H). Confirm enantiomeric enrichment of Isomer 2 by chiral analytical SFC, 98% ee, /(R>: 2.03 minutes; column: CHIRALCEL® OD-H, 4.6 x 150 mm; eluting with a mobile phase of 30% MeOH (0.2% IP A) in C02; column temperature: 40 °C; flow rate: 5 mL/minute; UV detection wavelength: 225 nm.
Alternate Preparation EXAMPLE 1B
Crystalline 5-(4-{2-[3-(Fluoromethyl)azetidin-l-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H- [l]benzopyrano[4,3-c]quinolin-2-ol, Isomer 2
Stir 5-(4-{2-[3-(fluoromethyl)azetidin-l-yl]ethoxy}phenyl)-8-(trifluoromethyl)-5H-[l]benzopyrano[4,3-c]quinolin-2-ol, 4-methylbenzenesulfonic acid, Isomer 2 (23.8 g, 0.034 mol) in water (250 mL) at 1000 rpm. Add NaOH (76 pL) and stir the solution for 2 hours. Add DCM (600 mL). Separate the mixture, dry the DCM extract with magnesium sulfate, filter the material through a syringe filter (0.45 pm), and concentrate to dryness. Allow the material to sit under a N2 stream over a weekend. Add 1 : 1 EtOH/water (80 mL) and stir the mixture with sonication. Collect a tan solid by filtration on a nylon membrane to give the title compound (10.47 g, 0.02 mol, 59%).
PAT
- Selective estrogen receptor degradersPublication Number: US-2023234960-A1Priority Date: 2018-07-12
- Selective estrogen receptor degraderPublication Number: CN-112424205-BPriority Date: 2018-07-12Grant Date: 2023-10-31
- selective estrogen receptor degraderPublication Number: CN-117379428-APriority Date: 2018-07-12
- Selective estrogen receptor degradersPublication Number: US-11993608-B2Priority Date: 2018-07-12Grant Date: 2024-05-28
- Selective estrogen receptor degradersPublication Number: US-12128040-B2Priority Date: 2018-07-12Grant Date: 2024-10-29
PAT
https://patents.google.com/patent/US11926634B2/en
Selective estrogen receptor degraders (SERDs) bind to the estrogen receptor (ER) and downregulate ER-mediated transcriptional activity. The degradation and downregulation caused by SERDs can be useful in the treatment of various proliferative immune mediated disorders, cell proliferation disorders, including cancers such as breast cancer, ovarian cancer, endometrial cancer, prostate cancer, uterine cancer, gastric cancer, and lung cancer as well as mutations due to emerging resistance. Some small molecule examples of SERDs have been disclosed in the literature (see, e.g., WO2005073204, WO2014205136, and WO2016097071). Nonetheless, there is a need for new SERDs to treat ER-positive cancers, such as breast cancer, gastric cancer, and/or lung cancer.
As described in U.S. Pat. No. 10,654,866 (the ‘866 patent) a series of SERDs of the following formula have been discovered, along with pharmaceutically acceptable salts thereof:
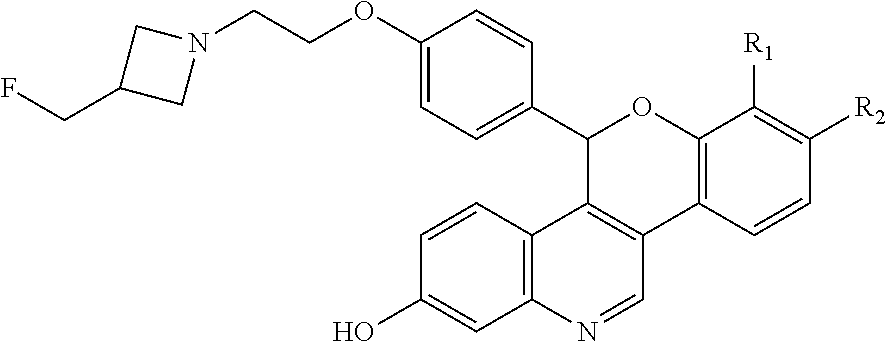
wherein one of R1 and R2 are independently Cl, F, —CF3, or —CH3, and the other is H.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Trade names | Inluriyo |
| Other names | LY3484356, LY-3484356 |
| AHFS/Drugs.com | Inluriyo |
| License data | US DailyMed: Imlunestrant |
| Routes of administration | By mouth |
| Drug class | Estrogen receptor antagonist |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2408840-26-4as tosylate: 2408840-41-3 |
| PubChem CID | 146603228 |
| DrugBank | DB19043 |
| ChemSpider | 115010421 |
| UNII | 9CXQ3PF69Uas tosylate: F7UDT90EW5 |
| KEGG | D12216as tosylate: D12217 |
| ChEMBL | ChEMBL5095183 |
| Chemical and physical data | |
| Formula | C29H24F4N2O3 |
| Molar mass | 524.516 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/218881s000lbl.pdf
- “FDA approves imlunestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer”. U.S. Food and Drug Administration (FDA). 25 September 2025. Retrieved 27 September 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - “U.S. FDA approves Inluriyo (imlunestrant) for adults with ER+, HER2-, ESR1-mutated advanced or metastatic breast cancer” (Press release). Eli Lilly. 25 September 2025. Retrieved 27 September 2025 – via PR Newswire.
- World Health Organization (2022). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 88”. WHO Drug Information. 36 (3). hdl:10665/363551.
Further reading
- Jhaveri, Komal L.; Jeselsohn, Rinath; Lim, Elgene; Hamilton, Erika P.; Yonemori, Kan; Beck, J. Thaddeus; et al. (June 2022). “A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Monotherapy results from EMBER”. Journal of Clinical Oncology. 40 (16_suppl): 1021. doi:10.1200/JCO.2022.40.16_suppl.1021. S2CID 249445691.
- Jhaveri, Komal; O’Shaughnessy, Joyce; Andre, Fabrice; Goetz, Matthew P.; Harbeck, Nadia; Martín, Miguel; et al. (March 2023). “Abstract OT1-01-02: EMBER-4: A phase 3 adjuvant trial of imlunestrant vs standard endocrine therapy (ET) in patients with ER+, HER2- early breast cancer (EBC) with an increased risk of recurrence who have previously received 2 to 5 years of adjuvant ET”. Cancer Research. 83 (5_Supplement): OT1–01–02-OT1-01–02. doi:10.1158/1538-7445.SABCS22-OT1-01-02.
- Neven, P.; Stahl, N.; Losada, M.J. Vidal; Jimenez, M. Martin; Kaufman, P.A.; Harbeck, N.; et al. (October 2023). “273P A preoperative window-of-opportunity (WOO) study of imlunestrant in ER+, HER2- early breast cancer (EBC): Final analysis from EMBER-2”. Annals of Oncology. 34: S292 – S293. doi:10.1016/j.annonc.2023.09.470. S2CID 264385454.
External links
- Clinical trial number NCT04975308 for “A Study of Imlunestrant, Investigator’s Choice of Endocrine Therapy, and Imlunestrant Plus Abemaciclib in Participants With ER+, HER2- Advanced Breast Cancer (EMBER-3)” at ClinicalTrials.gov
/////////Imlunestrant, FDA 2025, APPROVALS 2025, Inluriyo, CANCER, LY3484356, LY 3484356, 9CXQ3PF69U














