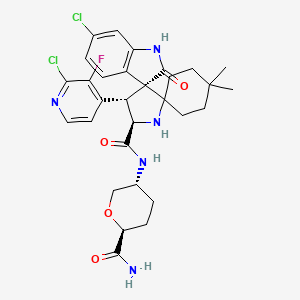
Milademetan
| Molecular Weight |
618.53 |
|---|---|
| Formula |
C30H34Cl2FN5O4 |
| CAS No. |
1398568-47-2 |
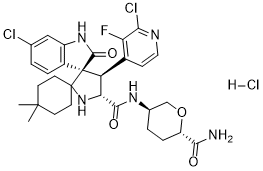
Milademetan. hcl
Chemical Formula: C30H35Cl3FN5O4
Exact Mass: 617.1972
Molecular Weight: 654.99
Elemental Analysis: C, 55.01; H, 5.39; Cl, 16.24; F, 2.90; N, 10.69; O, 9.77
1398568-47-2 (free base) 1398569-75-9 (tosylate) 2095625-97-9 (tosylate hydrate) Milademetan HCl
DS3032b; DS-3032b; DS 3032b; DS3032; DS-3032; DS 3032; DS-3032b tosylate; Milademetan tosylate; Milademetan HCl
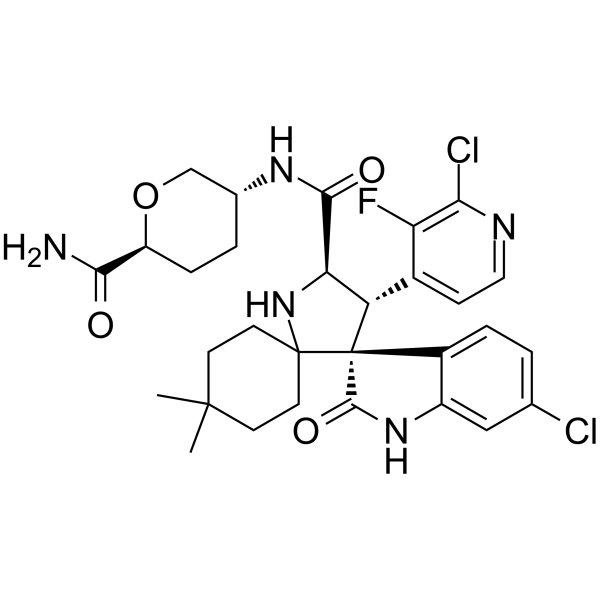
(3’R,4’S,5’R)-N-[(3R,6S)-6-carbamoyloxan-3-yl]-6”-chloro-4′-(2-chloro-3-fluoropyridin-4-yl)-4,4-dimethyl-2”-oxo-1”,2”-dihydrodispiro[cyclohexane-1,2′-pyrrolidine-3′,3”-indole]-5′-carboxamide hydrochloride
orphan drug, UNII:R3I80TLN7S, миладеметан , ميلاديميتان , 米拉美坦
(3’R,4’S,5’R)-N-((3R,6S)-6-Carbamoyltetrahydro-2H-pyran-3-yl)-6”-chloro-4′-(2-chloro-3-fluoro-4-pyridinyl)-4,4-dimethyl-2”-oxo-1”,2”-dihydrodispiro(cyclohexane-1,2′-pyrrolidine-3′,3”-indole)-5′-carboxamide
milademetan
rolontis
SPI-2012
Milademetan, also known as DS-3032b or DS-3032, is a potent and selective MDM2 inhibitor with potential antineoplastic activity. Upon oral administration, MDM2 inhibitor DS-3032b binds to, and prevents the binding of MDM2 protein to the transcriptional activation domain of the tumor suppressor protein p53. By preventing this MDM2-p53 interaction, the proteosome-mediated enzymatic degradation of p53 is inhibited and the transcriptional activity of p53 is restored. This results in the restoration of p53 signaling and leads to the p53-mediated induction of tumor cell apoptosis.
DS-3032 (Milademetan) is an orally available, potent and selective inhibitor of the p53-MDM2 (murine double minute 2) interaction. Milademetan binds to, and prevents the binding of MDM2 protein to the transcriptional activation domain of the tumor suppressor protein p53. Milademetan is 10-fold more potent than the first-generation inhibitor nutlin-3a. By preventing this MDM2-p53 interaction, the proteasome-mediated enzymatic degradation of p53 is inhibited and the transcriptional activity of p53 is restored. This results in the restoration of p53 signaling and leads to the p53-mediated induction of tumor cell apoptosis. DS-3032 is currently being evaluated in three phase 1 clinical trials for solid and hematological malignancies, including acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myeloid leukemia (CML) in blast phase, lymphoma and myelodysplastic syndrome (MDS).
- OriginatorRigel Pharmaceuticals
- DeveloperDaiichi Sankyo Inc; National Cancer Center Hospital East; Rain Therapeutics; University of Texas M. D. Anderson Cancer Center
- ClassAntineoplastics; Cyclohexanes; Indoles; Pyrrolidines; Small molecules
- Mechanism of ActionProto-oncogene protein c mdm2 inhibitors
- Orphan Drug StatusYes – Liposarcoma
- Phase IIILiposarcoma
- Phase IISarcoma; Solid tumours
- Phase I/IIAcute myeloid leukaemia
- Phase ILymphoma; Myelodysplastic syndromes
- PreclinicalMesothelioma
- No development reportedMultiple myeloma
- 10 Aug 2022Rain Therapeutics completes enrolment in phase-III clinical trials in Liposarcoma in (Inoperable/Unresectable, Metastatic disease, Second-line therapy or greater) in United Kingdom, Taiwan, Spain, Poland, South Korea, Italy, Hong Kong, Germany, Georgia, France, Canada, Belgium, Austria (PO) (NCT04979442)
- 09 Jun 2022Efficacy, adverse events and pharmacodynamics data from phase I/II trial in Acute myeloid leukemia presented at the 27th Congress of the European Haematology Association(EHA-2022)
- 04 May 2022Rain Therapeutics plans a phase I/II MANTRA-4 trial in Solid tumours (Combination therapy, Late-stage disease) in Second half of 2022
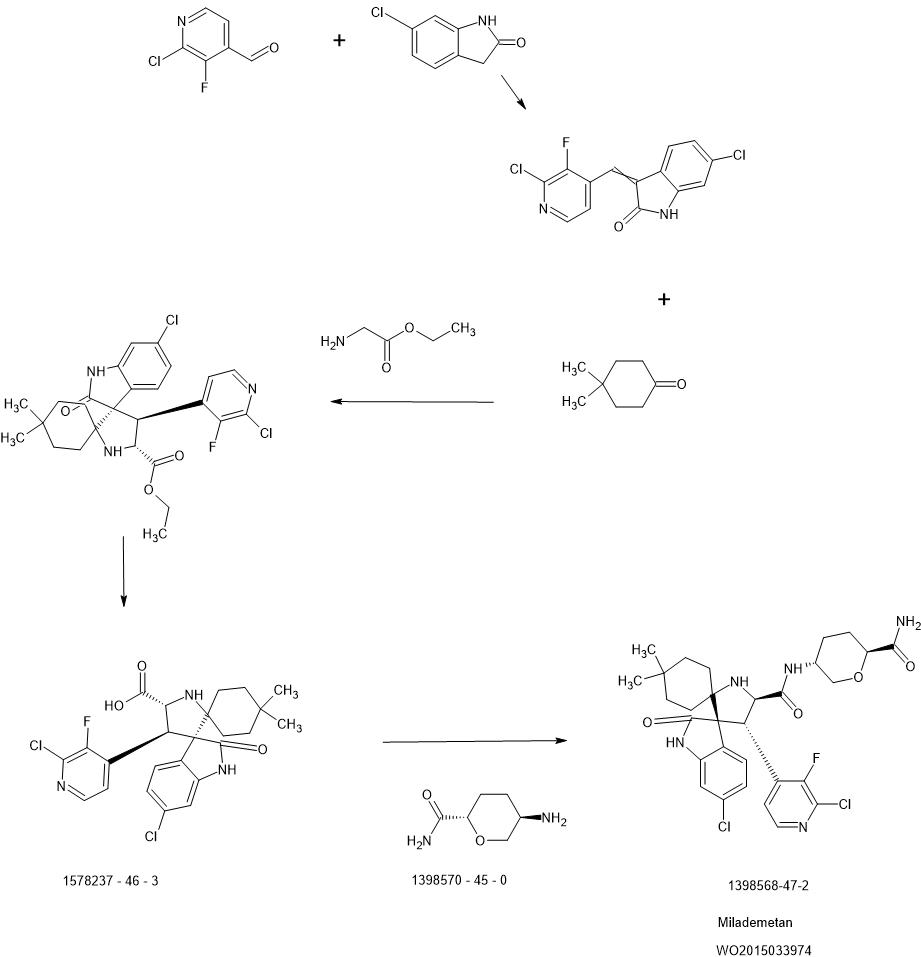
PATENT
WO2015033974
https://patentscope.wipo.int/search/en/detail.jsf?docId=JP274263016&_cid=P10-L9H0TY-72193-1
Ethyl (3’R,4’S,5’R)-6”-chloro-4′-(3-chloro-2-fluorophenyl)-4,4-dimethyl-2”-oxo 1″,2″-dihydrodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indole]-5′-carboxylate
1H NMR (500 MHz, CDCl3): δ = 0.67 (s, 3H), 0.91 (s, 3H), 1.10-1.19 (m, 2H), 1.17 (t, J=7.3 Hz, 3H), 1.25-1.33 (m, 1H), 1.44- 1.72 (m, 3H), 1.87-2.01 (m, 1H), 3.16 (s, 1H), 4.07-4.21 (m, 2H), 4.52 (d, J = 8.5 Hz, 1H), 4.83 (d, J = 8.5 Hz, 1H), 6.74 (d, J = 1.5Hz, 1H), 6.81-6.86 (m, 1H), 7.06 (dd, J = 8.3, 2.8 Hz, 1H), 7.10-7.16 (m, 1H), 7.37 (dd, J = 8.3, 1.8 Hz, 1H), 7.48-7.54 (m, 1H), 7.81 (s, 1H).
(HPLC conditions for optical purity determination)
カラム: CHIRALPAK OD-3R 4.6 × 150 mm, 3μm
Moving layer: 10mM Rinic acid buffer: MeCN = 40:60
Flow rate: 1.0 min/min
カラム Temperature: 40°C
Exhaust wavelength: 254 nm
Injection volume: 5 μL
Hold time: Labeling compound = 13.8 min, エナンチオマー= 12.9 min
11-1) Effects of various asymmetric catalysts
(HPLC conditions for measuring optical purity)
Column: CHIRALPAK OD-3R 4.6 × 150 mm, 3 µm
Mobile phase: 10 mM phosphoric acid buffer: MeCN = 40:60
Flow rate: 1.0 min/min
column Temperature: 40°C
Detection wavelength: 254 nm
Injection volume: 5 µL
Retention time: Title compound = 13.8 min, enantiomer = 12.9 min
Main results are shown in Table 1.
Table 2 shows the main results.
PATENT
WO2014038606
WO2014038606 CLICK HERE
-Carboxamide The compound (35 mg, 0.24 mmol) obtained in Reference Example 2, Step 3 was added to a solution of the compound (100 mg, 0.20 mmol) obtained in Step 3 of Reference Example 1 in N,N-dimethylformamide (4 ml). , triethylamine (0.04 ml, 0.30 mmol), 1-hydroxybenzotriazole (27 mg, 0.20 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46 mg, 0.24 mmol) were added. , and stirred for 1 hour at 50° C. After allowing to cool, the reaction solution was diluted with ethyl acetate, washed successively with water, saturated aqueous sodium hydrogencarbonate solution and saturated brine, and dried over anhydrous sodium sulfate. After evaporating the solvent under reduced pressure, the residue was purified by NH-silica gel column chromatography [chloroform:methanol=50:1 (v/v)]. After stirring for 24 hours at rt, the solvent was distilled off under reduced pressure to obtain 94 mg (76%) of the title compound as a solid.1H
– NMR (400 MHz, CDCl3 ) .) δ: 0.68 (3H, s), 0.95 (3H, s), 1.11-1.27 (2H, m), 1.35-1.81 (8H, m), 2.10-2.17 (1H, m), 2.25-2.32 (1H, m), 3.15(1H,t,J=10.5Hz), 3.27(1H,br s), 3.80(1H,dd,J=11.0,2.3Hz), 3.85-3.95(1H,m), 4.13(1H, ddd,J=10.8,4.5,1.3Hz),4.44(1H,d,J=9.2Hz),4.64(1H,d,J=9.2Hz),5.46(1H,d,J=3.7Hz),6.49( 1H,d,J=3.7Hz), 6.74(1H,d,J=1.8Hz), 7.07(1H,dd,J=8.2,1.8Hz), 7.31(1H,dd,J=8.2,2.3Hz), 7.48-7.52(2H,m),7.62(1H,s),8.05(1H,d,J=5.5Hz).MS
(ESI)m/z:618(M+H) +
6-chloro -1,3-dihydro-2H-indol-2-one (2.20 g, 13.11 mmol) and 2-chloro-3-fluoroisonicotinaldehyde (2.20 g, 13.8 mmol) in methanol (130 ml). , N,N-diisopropylethylamine (0.46 ml, 2.63 mmol) was added, and the mixture was heated under reflux for 16 hours. After standing to cool, the precipitate was collected by filtration, washed with cold methanol and dried to obtain 3.37 g (83%) of the title compound as a solid.
MS(APCI) m/z: 309(M+H) + .
-dione Under a nitrogen atmosphere, the compound obtained in Step 1 (1.86 g, 6.00 mmol), (5R,6S )-5,6-diphenylmorpholin-2-one (1.67 g, 6.60 mmol) and 4,4-dimethylcyclohexanone (0.83 g, 6.60 mmol) in tetrahydrofuran (30 ml) was added with diethyl boron trifluoride. An ether complex (0.15 ml, 1.20 mmol) and molecular sieve 4A (powder) (3 g) were added, and the mixture was heated and stirred at 70° C. for 7 days. After allowing to cool, insoluble matter was removed by filtration through celite, and the filtrate was washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure and purified by silica gel column chromatography [n-hexane:ethyl acetate=4:1→1:1 (v/v)] to obtain 3.39 g (84%) of the title compound as a solid. rice field.
1 H-NMR (400 MHz, CDCl3) δ: 0.21 (3H, s), 0.53 (3H, s), 0.89-1.08 (3H, m), 1.28-1.43 (3H, m), 1.73-1.81 (1H, m), 2.23-2.33 (1H, m), 4.58 (1H, d, J = 11.0Hz), 4.86 (1H, d, J = 3.2Hz), 5.31 (1H, d, J = 11.0Hz), 6.25 (1H, d, J = 8.3Hz) ,6.67(1H,dd,J=8.3,1.8Hz),6.72-6.77(2H,m),6.93(1H,d,J=1.8Hz),7.04-7.17(6H,m),7.18-7.25(3H ,m),7.79(1H,t,J=4.6Hz),7.99(1H,s),8.29(1H,d,J=5.0Hz).MS
(APCI)m/z:670(M+H) + .
The compound obtained in step 2 (630 mg, 0.94 mmol) was treated with acetonitrile (10 ml). Dissolve in water (4 ml), add potassium carbonate (130 mg, 0.94 mmol) and heat under reflux for 16 hours at 85° C. After allowing to cool, add anhydrous magnesium sulfate (113 mg, 0.94 mmol) and stir at room temperature for 15 minutes. After extraction with ethyl acetate, the organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. (2-chloro-3-fluoropyridin-4-yl)-1′-[(1R,2S)-2-hydroxy-1,2-diphenylethyl]-4,4-dimethyl-2″-oxo-1″ ,2″-dihydrodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indole]-5′-carboxylic acid (650 mg, 100%) was obtained as a solid [MS (ESI) m/z :688(M+H) +]. The resulting carboxylic acid (650 mg, 0.94 mmol) was dissolved in methanol (30 ml) and water (8 ml), and diammonium cerium (IV) nitrate (1.55 g, 2.82 mmol) was added under ice-cooling. Stir at room temperature for 30 minutes. Potassium carbonate (780 mg, 5.64 mmol) was added under ice-cooling, and the mixture was stirred at the same temperature for 1 hour. After removing the insoluble matter by filtration through celite, the filtrate was concentrated under reduced pressure, water was added to the resulting residue, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography [chloroform:methanol=20:1→4:1 (v/v)] to obtain 152 mg (33%) of the title compound as a solid. .
1 H-NMR (500 MHz, CD 3 OD) δ: 0.74 (3H, s), 0.9 (3H, s), 1.29-1.44 (2H, m), 1.48-1.58 (2H, m), 1.64-1.76 (1H ,m),1.94-2.02(1H,m),2.11(1H,ddd,J=14.0,14.0,4.0Hz),2.43-2.53(1H,m),5.07(1H,d,J=10.3Hz), 5.32(1H,d,J=10.3Hz),6.84(1H,d,J=1.7Hz),7.16(1H,dd,J=8.3,2.0Hz),7.63(1H,dd,J=8.0,2.3Hz) ),7.75(1H,t,J=5.2Hz),8.15(1H,d,J=5.2Hz).
MS(ESI)m/z:492(M+H) + .
-hexonate 2,6-anhydro-3,4,5-trideoxy-5-( dibenzylamino)-L-erythro-hexonate methyl 2,6-anhydro-3,4,5-trideoxy-5-(dibenzylamino)-L-erythro-hexonate (1.60 g, 4.70 mmol) was The mixture was dissolved in methanol (30 ml), 1N aqueous sodium hydroxide solution (10 ml) was gradually added under ice-cooling, and the mixture was stirred at room temperature for 3 hours. Dowex 50W-X8 was added to the reaction mixture to adjust the pH to 5 to 6, insoluble materials were removed by filtration, and the filtrate was concentrated under reduced pressure to obtain 1.7 g (100%) of the title compound as a solid.
1 H-NMR (400 MHz, CDCl 3 ) δ: 1.18-1.26(1H,m), 1.36-1.48(1H,m), 1.79-1.97(2H,m), 2.62(1H,t,J=11.0Hz) ,3.18(1H,t,J=10.4Hz),3.40(1H,d,J=11.5Hz),3.51-3.61(4H,m),3.90-3.99(1H,m),7.12-7.38(10H,m ).
MS(ESI)m/z:326(M+H) + .
The compound (870 mg, 2.67 mmol) obtained in Step 1 above was dissolved in N,N-dimethylformamide (30 ml). 1-hydroxybenzotriazole (361 mg, 2.67 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (614 mg, 3.20 mmol) were added and stirred at room temperature for 15 minutes. Ammonium chloride (285 mg, 5.44 mmol) and N,N-diisopropylethylamine (1.86 ml, 10.7 mmol) were added and stirred at room temperature for 8 hours. After diluting with ethyl acetate, the organic layer was washed with saturated aqueous sodium hydrogencarbonate solution and saturated brine in that order, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to give 495 mg (57%) of the title compound as a solid.
1 H-NMR (400 MHz, CDCl 3 ) δ: 1.35-1.45 (1H, m), 1.60-1.70 (1 H, m), 2.10-2.18 (1 H, m), 2.21-2.28 (1 H, m), 2.76 ( 1H,tt,J=11.4,4.0Hz),3.44(1H,t,J=10.9Hz),3.67(4H,q,J=14.2Hz),3.71-3.73(1H,m),4.04(1H,dq ,J=11.0,2.1Hz),5.35(1H,s),6.40(1H,s),7.21-7.36(10H,m).MS
(ESI)m/z:325(M+H) + .
The compound (490 mg, 1.51 mmol) obtained in Step 2 above was dissolved in ethanol (10 ml) and treated with 20% palladium hydroxide. (100 mg) was added, and the mixture was stirred at room temperature for 16 hours under a hydrogen atmosphere. After removing the catalyst by filtration through celite, the filtrate was distilled off under reduced pressure and dried to obtain 215 mg (99%) of the title compound as a solid.
1 H-NMR (400 MHz, DMSO-d 6 ) δ: 1.11-1.22(1H,m), 1.25-1.35(1H,m), 1.83-1.91(2H,m), 2.51-2.60(1H,m), 2.90(1H,t,J=10.5Hz),3.52(1H,d,J=11.9Hz),
3.78-3.84 (1H,m),6.99(1H,br s),7.09(1H,br s). (ESI) m/z: 145(M+H) + .
PATENT
WO2012121361
PATENT
WO2015033974
PAPER
https://pubs.acs.org/doi/10.1021/acs.oprd.2c00192
Abstract

Herein, we report the structure and synthesis of the potent MDM2-p53 inhibitor BI-0282. The complex spirooxindole scaffold bearing four stereocenters embedded in a rigid polycyclic ring-system was effectively prepared on a multi-gram scale in only five synthesis steps employing a three-component 1,3-dipolar cycloaddition and a late-stage Davis–Beirut reaction as key steps.
Compound 1
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Milademetan is under investigation in clinical trial NCT02319369 (Safety, Tolerability and Pharmacokinetics of Milademetan Alone and With 5-Azacitidine (AZA) in Acute Myelogenous Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS)).
- [1]. ARYL SULFONOHYDRAZIDES. WO 2017069289 A1.[2]. M.M. Gounder, et al. Milademetan, an oral MDM2 inhibitor, in well-differentiated/dedifferentiated liposarcoma: results from a phase 1 study in patients with solid tumors or lymphomas. European Journal of Cancer 138S2 (2020) S1–S62.
/////////Milademetan, DS3032b, DS-3032b, DS 3032b, DS3032, DS-3032, DS 3032, DS-3032b tosylate, Milademetan tosylate, Milademetan HCl, orphan drug, UNII:R3I80TLN7S, миладеметан , ميلاديميتان , 米拉美坦
CC1(C)CCC2(CC1)N[C@H]([C@H](C1=C(F)C(Cl)=NC=C1)[C@]21C(=O)NC2=CC(Cl)=CC=C12)C(=O)N[C@@H]1CC[C@H](OC1)C(N)=O