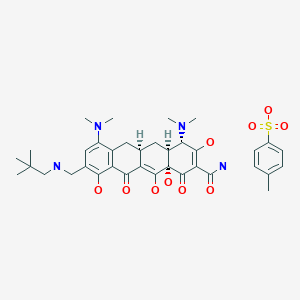
Omadacycline tosylate
728.8521, C29H40N4O7. C7H8O3S
CAS: 1075240-43-5
389139-89-3 FREE FORM
FDA 2018/10/3, Nuzyra
オマダサイクリントシル酸塩;
UNII-5658Y89YCD
Omadacycline has been used in trials studying the treatment of Bacterial Pneumonia, Bacterial Infections, Community-Acquired Infections, and Skin Structures and Soft Tissue Infections. Omadacycline represents a significant advance over the well-known tetracycline family, and has been shown to be highly effective in animal models at treating increasingly problematic, clinically prevalent infections caused by gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), and by gram-negative, atypical and anaerobic bacteria, including those resistant to currently available classes of antibiotics and known to cause diseases such as pneumonias, urinary tract infections, skin diseases and blood-borne infections in both the hospital and community settings.
Omadacycline (formerly known as PTK-0796)[1] is a broad spectrum antibiotic belonging to the aminomethylcycline subclass[2] of tetracycline antibiotics. In the United States, it was approved in October 2018 for the treatment of community-acquired bacterial pneumonia and acute skin and skin structure infections.
In vitro studies
In vitro studies have shown that omadacycline has activity against a broad range of Gram-positive and select Gram-negativepathogens.[3] Omadacycline has potent in vitro activity against Gram-positive aerobic bacteria including methicillin-resistant Staphylococcus aureus (MRSA), pencillin-resistant and multi-drug resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus. Omadacycline also has antimicrobial activity against common Gram-negative aerobes, some anaerobes, and atypical bacteria such as Legionella and Chlamydia.[4] This activity translated to potent efficacy for omadacycline in an in vivo systemic infection model in mice.[5]
Additional in vitro and in vivo studies of omadacycline metabolism, disposition, and drug interactions show that omadacycline is metabolically stable (i.e., it does not undergo significant biotransformation) and neither inhibits nor interacts with metabolizing enzymes or transporters.[6]
Mechanism of action
The mechanism of action of omadacycline is similar to that of other tetracyclines – inhibition of bacterial protein synthesis. Omadacycline has activity against bacterial strains expressing the two main forms of tetracycline resistance (efflux and ribosomal protection).[7]
Clinical trials
A phase 2 study was conducted comparing the safety and efficacy of omadacycline to linezolid for the treatment of complicated skin and skin structure infections. Patients were randomized at 11 sites in the US to receive either omadacycline 100 mg intravenously once daily with an option to transition to 200 mg orally once daily or linezolid 600 mg intravenously twice daily with an option to transition to 600 mg orally twice daily. The results indicated that omadacycline is well-tolerated and has the potential to be an effective treatment in patients with complicated skin and skin structure infections.[8]
In June 2013, the US Food and Drug Administration (FDA) designated the intravenous and oral formulations of omadacycline as a qualified infectious disease product in the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia.[9]
A 650 patient phase 3 registration study comparing omadacycline to linezolid for the treatment of acute bacterial skin and skin structure infections began in June 2015.[10][11]Omadacycline met the primary efficacy endpoint of early clinical response with statistical non-inferiority (10% margin) compared to linezolid, and was generally safe and well-tolerated. The most common treatment-emergent adverse events were gastrointestinal side effects (18.0% for omadacycline vs. 15.8% for linezolid).[12]
A 750 patient phase 3 study comparing omadacycline to moxifloxacin for the treatment of community-acquired bacterial pneumonia began in November 2015.[13] Omadacycline was statistically non-inferior to moxifloxacin at the early clinical response, 72 to 120 hours after therapy was initiated.[14]
In May 2016, a phase 1b study of omadacycline in urinary tract infection was initiated.[15]
In August 2016, a second phase 3 study of omadacycline was initiated in patients with acute bacterial skin and skin structure infections, comparing the efficacy and safety of once-daily, oral omadacycline to that of twice-daily, oral linezolid.[16] In July 2017, analysis of the data showed that all of the primary and secondary endpoints required for submission to the FDA and EMA were met. This was the third phase 3 registration study of omadacycline with favorable results.[17]
Discovery
Omadacycline was invented at Tufts University School of Medicine by a research team led by Mark L. Nelson with Mohamed Ismail while at Tufts and Kwasi Ohemeng and Laura Honeyman at Paratek Pharmaceuticals, Boston. The team applying their chemistry methods to the tetracycline scaffolds created over 3000 new derivatives, leading to the novel third generation compounds omadacycline and sarecycline. 18[18]
PAPERS
Tetrahedron Letters (2008), 49(42), 6095-6100
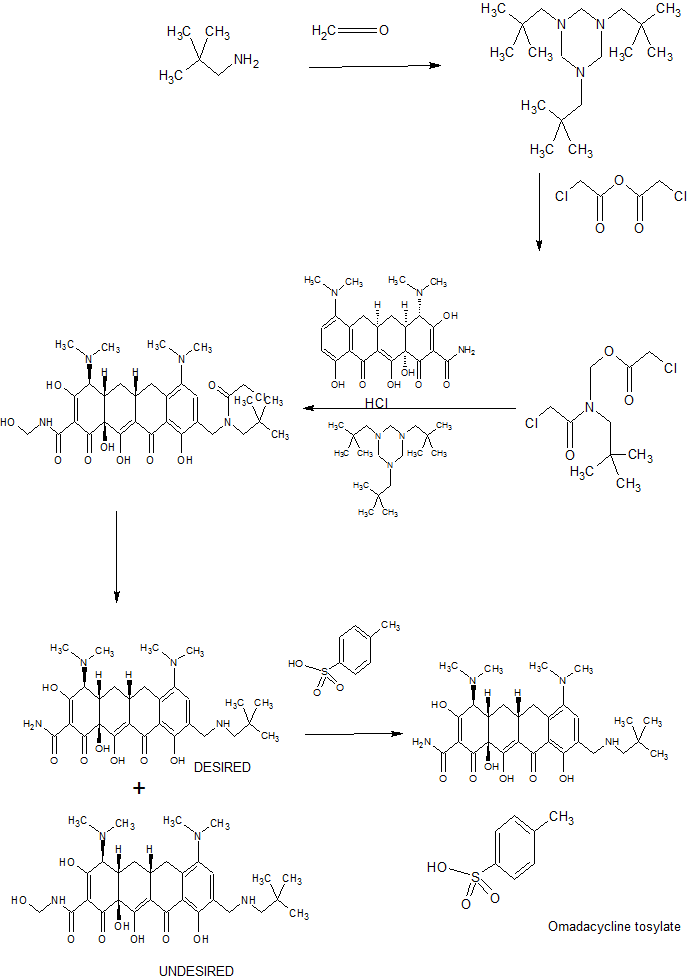
PATENTS
WO 2009120389
WO 2009111064
WO 2017165729
WO 2018026987
WO 2018085216
SYNTHESIS BY PHARMACODIA WEBSITE
Omadacycline, www.pharmacodia.com
REF Omadacycline, www.pharmacodia.com
References
- Jump up^ Boggs, Jennifer. “Antibiotic Firm Paratek Joins IPO Queue; Aiming for $92M”. bioworld.com. Clarivate Analytics. Retrieved October 17, 2017.
- Jump up^ Honeyman, Laura; Ismail, Mohamed; Nelson, Mark L.; Bhatia, Beena; Bowser, Todd E.; Chen, Jackson; Mechiche, Rachid; Ohemeng, Kwasi; Verma, Atul K.; Cannon, E. Pat; MacOne, Ann; Tanaka, S. Ken; Levy, Stuart (2015). “Structure-Activity Relationship of the Aminomethylcyclines and the Discovery of Omadacycline”. Antimicrobial Agents and Chemotherapy. 59 (11): 7044–7053. doi:10.1128/AAC.01536-15. PMC 4604364. PMID 26349824.
- Jump up^ Tanaka, S. Ken (20 June 2016). “In Vitro and In Vivo Assessment of Cardiovascular Effects with Omadacycline”. Antimicrobial Agents and Chemotherapy. 60 (9): 5247–53. doi:10.1128/AAC.00320-16. PMC 4997885. PMID 27324778.
- Jump up^ Villano, Stephen (19 August 2016). “Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections”. Future Microbiology. 11: 1421–1434. doi:10.2217/fmb-2016-0100. Retrieved 24 August 2016.
- Jump up^ MacOne, A. B.; Caruso, B. K.; Leahy, R. G.; Donatelli, J.; Weir, S.; Draper, M. P.; Tanaka, S. K.; Levy, S. B. (February 2014). “In Vitro and in Vivo Antibacterial Activities of Omadacycline, a Novel Aminomethylcycline”. Antimicrobial Agents and Chemotherapy. 58 (2): 1127–1135. doi:10.1128/AAC.01242-13. PMC 3910882. PMID 24295985.
- Jump up^ Flarakos, Jimmy (8 August 2016). “Clinical disposition, metabolism and in vitro drug–drug interaction properties of omadacycline”. Xenobiotica: 1–15. doi:10.1080/00498254.2016.1213465.
- Jump up^ Draper, M. P.; Weir, S.; MacOne, A.; Donatelli, J.; Trieber, C. A.; Tanaka, S. K.; Levy, S. B. (March 2014). “Mechanism of Action of the Novel Aminomethylcycline Antibiotic Omadacycline”. Antimicrobial Agents and Chemotherapy. 58 (3): 1279–1283. doi:10.1128/AAC.01066-13. PMC 3957880. PMID 24041885.
- Jump up^ Noel, G. J.; Draper, M. P.; Hait, H.; Tanaka, S. K.; Arbeit, R. D. (November 2012). “A Randomized, Evaluator-Blind, Phase 2 Study Comparing the Safety and Efficacy of Omadacycline to Those of Linezolid for Treatment of Complicated Skin and Skin Structure Infections”. Antimicrobial Agents and Chemotherapy. 56 (11): 5650–5654. doi:10.1128/AAC.00948-12. PMC 3486554. PMID 22908151.
- Jump up^ “Paratek Pharmaceuticals Announces FDA Grant of Qualified Infectious Disease Product (QIDP) Designation for Its Lead Product Candidate, Omadacycline”. prnewsire.com. PR Newswire. January 3, 2013. Retrieved October 17, 2017.
- Jump up^ Seiffert, Don (2015). “Paratek presents new trial data for antibiotic as late-stage trials continue”. bizjournals.com. American City Business Journals. Retrieved October 17,2017.
- Jump up^ “Omadacycline Versus Linezolid for the Treatment of ABSSSI (EudraCT #2013-003644-23)”. clinicaltrials.gov. Retrieved 2015-10-13.
- Jump up^ “Paratek Announces that Omadacycline Met All Primary and Secondary Efficacy Outcomes Designated by FDA and EMA in a Phase 3 Study in Acute Bacterial Skin Infections; Omadacycline was Generally Safe and Well-Tolerated”. finance.yahoo.com. Retrieved 3 July 2016.
- Jump up^ “Omadacycline vs Moxifloxacin for the Treatment of CABP (EudraCT #2013-004071-13)”. clinicaltrials.gov. Retrieved 2015-10-13.
- Jump up^ “Paratek Announces Positive Phase 3 Study of Omadacycline in Community-Acquired Bacterial Pneumonia”. www.globenewswire.com. April 3, 2017. Retrieved 16 May 2017.
- Jump up^ “Paratek Initiates Phase 1b Study of Omadacycline in Urinary Tract Infection”. globenewswire.com. May 2, 2016. Retrieved 3 July 2016.
- Jump up^ “Paratek Initiates Phase 3 Study of Oral-only Omadacycline in ABSSSI”. globenewswire.com. August 15, 2016. Retrieved 15 August 2016.
- Jump up^ “Paratek Announces Phase 3 Study of Oral-Only Dosing of Omadacycline Met All Primary and Secondary FDA and EMA Efficacy Endpoints in Acute Bacterial Skin Infections”. www.globenewswire.com. July 17, 2017. Retrieved 19 July 2017.
- Jump up^ Ref: Mark L. Nelson and Kwasi Ohemeng: 4-dedimethylamino tetracycline compounds, United States (US) patent number 7,056,902 (2006)
 |
|
| Clinical data | |
|---|---|
| Trade names | Nuzyra |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C29H40N4O7 |
| Molar mass | 556.66 g·mol−1 |
| 3D model (JSmol) | |
/////////////FDA 2018, Nuzyra, Omadacycline tosylate, Omadacycline, オマダサイクリントシル酸塩 ,PTK-0796, PTK 0796
CC1=CC=C(C=C1)S(O)(=O)=O.[H][C@@]12CC3=C(C=C(CNCC(C)(C)C)C(O)=C3C(=O)C1=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@@H](N(C)C)[C@]1([H])C2)N(C)C