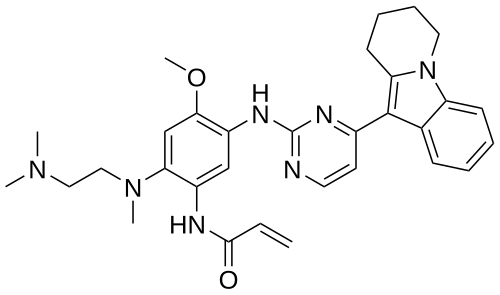
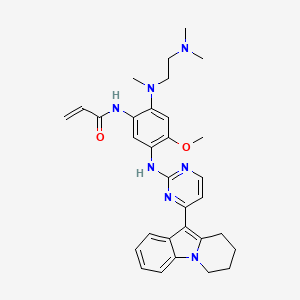
Oritinib
- CAS 2035089-28-0
- MESYLATE CAS 2180164-79-6
- SH-1028
- SK593H37SC
- N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide
- 539.7 g/mol, C31H37N7O2
- rilertinib
CHINA 2024, Nanjing Sanhome Pharmaceutical.
N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide
Oritinib is an investigational new drug currently under investigation for its potential use in cancer treatment.[1][2] As a epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, oritinib targets specific enzymes involved in the signaling pathways that regulate cell division and survival, which are often dysregulated in cancer cells.[1]
Oritinib (SH-1028), an irreversible third-generation EGFR TKI, overcomes T790M-mediated resistance in non-small cell lung cancer. Oritinib (SH-1028), a mutant-selective inhibitor of EGFR kinase activity, inhibits EGFRWT, EGFRL858R, EGFRL861Q, EGFRL858R/T790M, EGFRd746-750 and EGFRd746-750/T790M kinases, with IC50s of 18, 0.7, 4, 0.1, 1.4 and 0.89 nM, respectively.
PAT
https://patents.google.com/patent/CN115974845B/en
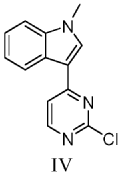
Reaction condition optimization experiment:
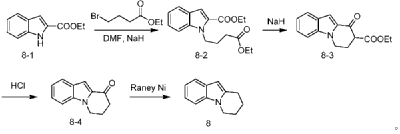
The experimental group numbered 1 referred to in table 1 below is the preparation of 1-methyl-3- (2-chloro-4-pyrimidinyl) indole, which was prepared as follows:
To a 10mL reaction tube, 2, 4-dichloropyrimidine (74.5 mg,0.05 mol), zinc triflate (67.3 mg,0.37 equiv), scandium triflate (7.4 mg,0.03 equiv) and 1-methylindole (78.6 mg,1.2 equiv) were added under inert gas atmosphere, and acetonitrile (2.5 mL) were heated to 80℃to react for 24 hours. The reaction was quenched with 30ml of ethyl acetate, the above mixture was added to a separating funnel, 50ml of saturated aqueous sodium carbonate and 50ml of saturated aqueous ammonium chloride were added thereto, and the mixture was shaken for 2 minutes, and the organic phase was taken after the liquid in the separating funnel had settled and separated. The aqueous phase was rinsed with 30ml of ethyl acetate under shaking for 2 times, the whole organic phase was collected, silica gel powder and anhydrous sodium sulfate were added thereto, and the mixture was dried under reduced pressure and packed into a silica gel column. Sequential gradient elution was performed using 250ml (PE: EA: triethylamine 16:4:1), 250ml (PE: EA: triethylamine 15:5:1), 250ml (PE: EA: triethylamine 40:20:3) as developing reagent. The eluent is collected and dried under reduced pressure to obtain pale yellow solid with the yield of 90 percent.
The nuclear magnetic resonance spectrum of 1-methyl-3- (2-chloro-4-pyrimidinyl) indole is as follows:
1H NMR(400MHz,DMSO-d6)δ8.51(d,J=5.9Hz,2H),8.40(dd,1H),7.82(d,J=5.4Hz,1H),7.56(dd,1H),7.28(pd,J=7.1,1.4Hz,2H),3.88(s,3H).
13C NMR(101MHz,DMSO)δ164.55,160.32,158.75,137.84,134.83,125.30,122.81,121.74,121.64,114.43,110.90,110.76,33.31.
PAT
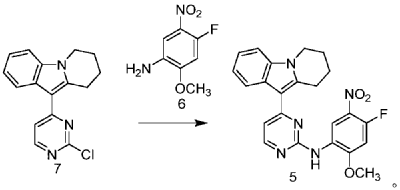
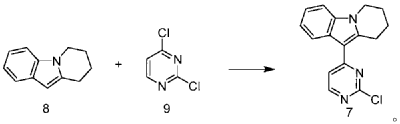
CN109705118
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN242181067&_cid=P20-MEGI3F-20821-1
| Step 1: Synthesis of 10-(2-chloropyrimidin-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indole |
| |
| In a 100L vertical jacketed glass reactor, add ethylene glycol dimethyl ether (39.15kg) and 2,4-dichloropyrimidine (3.915kg). Cool the solid-liquid mixture to below 10°C, then add anhydrous aluminum chloride (3.855kg) in batches, controlling the addition rate to keep the temperature below 30°C. After the addition is complete, stir at 25±5°C for 30 minutes, then add 6,7,8,9-tetrahydropyrido[1,2-a]indole (4.500kg). Raise the temperature to 60±5°C and react for 3 hours. Monitor by HPLC until the 6,7,8,9-tetrahydropyrido[1,2-a]indole content does not exceed 1.0%, confirming the reaction is complete. The reaction solution was cooled to below 25° C., purified water (90.0 kg) was added, stirred, and filtered. The filter cake was added to acetonitrile (17.8 kg), slurried, filtered, and dried to obtain a yellow powdery solid, a total of 6.652 kg, with a yield of 89.2%. |
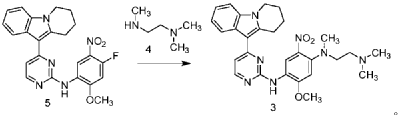
| Step 2: Synthesis of N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-amine |
| |
| To a 500L glass-lined reactor, sec-butyl alcohol (80.82kg), 10-(2-chloropyrimidin-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indole (6.652kg), 4-fluoro-2-methoxy-5-nitroaniline (4.363kg), and p-toluenesulfonic acid monohydrate (4.816kg) were added to obtain a solid-liquid mixture. The reaction mixture was heated to reflux, and the solid gradually dissolved. As the reaction proceeded, a yellow solid precipitated. After reflux for 7.5 hours, the reaction was monitored by HPLC to confirm completion. Heating was stopped, the reaction mixture was cooled to below 15°C, stirred for 1 hour, and the solid was centrifuged and filtered. Acetonitrile (31.5kg) was added to the filter cake, and the mixture was slurried at 25±5°C for 1.5 hours. The mixture was centrifuged and dried to obtain the title compound, a total of 9.548kg, with a yield of 94.0%. |
| Step 3: Synthesis of N 1 -(2-dimethylaminoethyl)-5-methoxy-N 1 -methyl-2-nitro-N 4 -(4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)phenyl-1,4-diamine |
| |
| To a 100 L vertical jacketed glass reactor, add N,N-dimethylacetamide (44.7 kg), N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-amine (9.548 kg), N,N,N’-trimethylethylenediamine (3.380 kg), and N,N-diisopropylethylamine (4.841 kg). Under nitrogen, the reaction mixture was reacted at 85±5°C for 2 hours and monitored by HPLC until the reaction was complete. The reaction solution was cooled to below 70°C, purified water (95.5 kg) was added, filtered, and dried to obtain the title compound, a total of 8.206 kg, with a yield of 72.2%. |
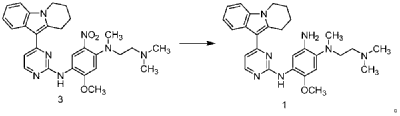
| Step 4: Synthesis of N 1 -(2-(dimethylamino)ethyl)-5-methoxy-N 1 -methyl-N 4 -(4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)benzene-1,2,4-triamine |
| |
| A 100 L vertical jacketed reactor was charged with anhydrous ethanol (32.39 kg), purified water (14.32 kg), N 1 -(2-dimethylaminoethyl)-5-methoxy-N 1 -methyl-2-nitro-N 4 -(4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)phenyl-1,4-diamine (4.103 kg), reduced iron powder (2.224 kg), and ammonium chloride (2.129 kg). The reaction mixture was refluxed for 1.5 hours and monitored by HPLC until the reaction was complete. The reaction mixture was cooled to below 50°C and filtered through diatomaceous earth to remove the solid. The filtrate was concentrated, and tetrahydrofuran (3.45 kg) and purified water (34.71 kg) were added to the residue. The mixture was slurried, filtered, and dried to obtain 3.244 kg of the title compound in an 84.0% yield. |
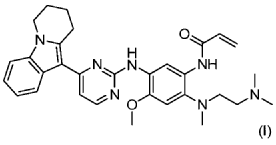
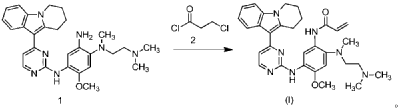
| Step 5: Synthesis of N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)allylamide |
| |
| Add N,N-dimethylacetamide (48.6 kg) to a 100 L vertical jacketed glass reactor. Raise the temperature to 40°C, then add N₁- ( 2-(dimethylamino)ethyl)-5-methoxy- N₁ -methyl- N₄- (4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)benzene-1,2,4-triamine (6.487 kg). Then, begin the dropwise addition of 3-chloropropionyl chloride (1.777 kg). Control the addition rate to no more than 60°C. After the addition is complete, cool the reaction mixture and stir at 40±5°C for 1 hour. Sample the mixture and monitor the reaction by HPLC until complete. Add purified water (0.253 kg) and stir for 30 minutes. |
| The reaction mixture was heated at 80±5°C, triethylamine (13.52 kg) was added, and the temperature was raised to 95±5°C. After reacting for 2 hours, the reaction was complete as determined by HPLC. The temperature was then lowered, and methanol (83.0 kg) was added. The mixture was then cooled and crystallized, filtered, and dried to obtain 4.953 kg of the title compound, with a yield of 68.6% and a purity of 97.37%. |
| Step 6: Purification of N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)allylamide |
| Anhydrous ethanol (31.25 kg) was added to a 100 L reactor and heated to above 70°C. The crude N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)allylamide prepared in step 5 was added. The reaction mixture was heated and stirred under nitrogen until dissolved. The reaction mixture was cooled to below 10°C, the precipitated solid was centrifuged and dried under vacuum at 60±5°C for more than 12 hours to obtain 4.559 kg of the title compound with a yield of 92.1% and a purity of 98.73%. 1 H NMR (300 MHz, DMSO-d 6 )δ10.20(s,1H),8.65(s,1H),8.34(d,1H),8.11(s,1H),8.06(d,1H),7.43(d, 1H),7.19-7.03(m,3H),6.98(s,1H),6.57-6.41(m,1H),6.28-6.15(m,1H),5.8 2-5.71(m,1H),4.09(t,2H),3.84(s,3H),3.18(t,2H),3.06-2.92(m,2H),2.66 (s,3H),2.47-2.40(m,2H),2.27(s,6H),2.08-1.96(m,2H),1.87-1.74(m,2H). ESI-Ms m/z: 540.3 [M+H] + . |
| Example 2: Synthesis of N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)allylamide |
| |
| The preparation method is the same as that in step 5 of Example 1, except that N,N-dimethylacetamide is replaced by N,N-dimethylformamide. The purity of the obtained title compound is 69%. |
| The N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)allylamide of the present invention prepared according to the above method has a high yield and purity, mild reaction conditions, easy purification, stable process, easy operation, environmental friendliness, and can meet the requirements of industrial-scale production and application. |
Syn
European Journal of Medicinal Chemistry 291 (2025) 117643
Oritinib represents a third-generation EGFR TKI engineered by Nanjing Sanhome Pharmaceutical. This agent specifically targets both EGFR-sensitizing mutations and the T790 M resistance mutation,
thereby addressing resistance mechanisms linked to prior-generation EGFR-TKIs. In 2024, the NMPA granted approval for Oritinib to treat adult patients with locally advanced or metastatic NSCLC who have experienced disease progression during or following EGFR-TKI therapy and possess confirmed EGFR T790 M mutation-positive status. The mechanism of action of Oritinib involves irreversible binding to mutant EGFR, including the T790 M variant, which in turn suppresses down stream signaling pathways responsible for tumor cell proliferation and survival [28]. The mechanism of Oritinib effectively inhibits tumor growth in patients harboring T790M-mediated resistance to first- and second-generation EGFR-TKIs. Clinical efficacy was established in a Phase II trial (NCT03823807) enrolling patients with EGFR T790 Mmutation-positive NSCLC who had experienced disease progression following prior EGFR-TKI therapy. This study documented an ORR of 60.5 % and a median PFS of 9.6 months, highlighting substantial anti
tumor efficacy in this specific patient cohort. In terms of safety, Oritinib exhibited favorable tolerability. The predominant treatment-related adverse events were rash, diarrhea, and elevated liver enzymes, pri
marily of mild (Grade 1) or moderate (Grade 2) severity. No dose-limiting toxicities were encountered, and the overall safety profile aligned with those observed for other third-generation EGFR-TKIs [29].
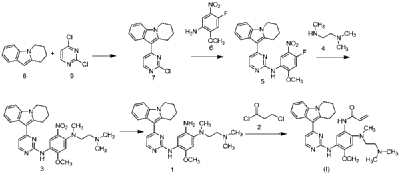
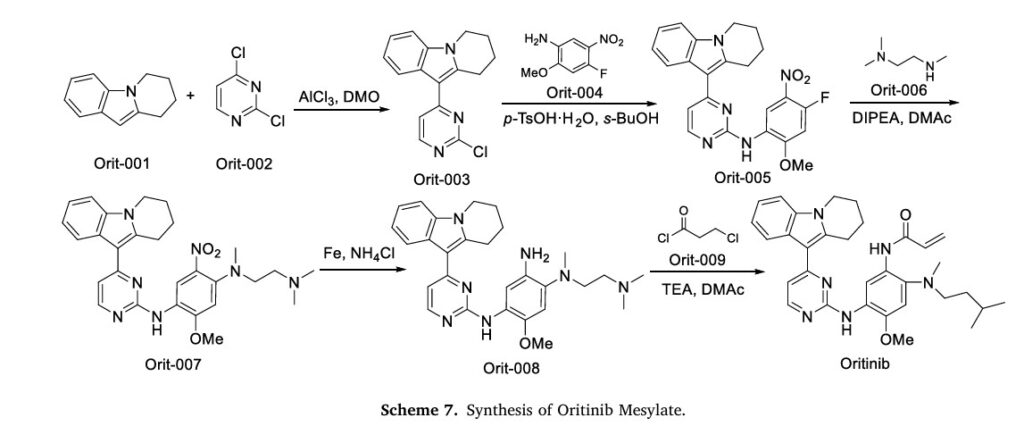
The synthetic route of Oritinib Mesylate, shown in Scheme 7, begins with nucleophilic substitution reaction between Orit-001 and Orit-002 to yield Orit-003, which further reacts with Orit-004 via nucleophilic substitution to produce Orit-005 [30]. Orit-005 subsequently undergoes another nucleophilic substitution with Orit-006 to generate Orit-007. Following this, Orit-007 is reduced to form Orit-008. Finally, an amidation reaction between Orit-008 and Orit-009 affords Oritinib.
[28] C. Zhou, A. Xiong, L. Miao, J. Chen, K. Li, H. Liu, Z. Ma, H. Wang, Z. Lu, J. Shen,
P51.03 oritinib (SH-1028), a third-generation EGFR-TKI in advanced NSCLC
patients with positive EGFR T790M: results of a single-arm phase Ib trial,
J. Thorac. Oncol. 16 (2021) S1119–S1120.
[29] C. Zhou, A. Xiong, J. Zhao, W. Li, M. Bi, J. Chen, K. Li, L. Miao, Y. Mao, D. Wang,
7MO oritinib (SH-1028) a third-generation EGFR tyrosine kinase inhibitor in
locally advanced or metastatic NSCLC patients with positive EGFR T790M: results
of a single-arm phase II trial, Ann. Oncol. 33 (2022) S31.
[30] L. Zhao, W. Fu, W. Wu, J. Liu, J. Jin, Method for Preparing Tricyclic Compound as
EGFR Kinase Inhibitor, 2019. CN109705118A.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Xiong A, Ren S, Liu H, Miao L, Wang L, Chen J, et al. (October 2022). “Efficacy and Safety of SH-1028 in Patients With EGFR T790M-Positive NSCLC: A Multicenter, Single-Arm, Open-Label, Phase 2 Trial”. Journal of Thoracic Oncology. 17 (10): 1216–1226. doi:10.1016/j.jtho.2022.06.013. PMID 35798241.
- “Rilertinib – Nanjing Sanhome Pharmaceutical”. AdisInsight. Springer Nature Switzerland AG.
| Clinical data | |
|---|---|
| Other names | SH-1028 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2035089-28-0 |
| PubChem CID | 122666966 |
| ChemSpider | 115007246 |
| UNII | SK593H37SC |
| Chemical and physical data | |
| Formula | C31H37N7O2 |
| Molar mass | 539.684 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
- New drugs approved by the NMPA in 2024: Synthesis and clinical applicationsPublication Name: European Journal of Medicinal ChemistryPublication Date: 2025-07-05PMID: 40262297DOI: 10.1016/j.ejmech.2025.117643
- Safety, efficacy, and pharmacokinetics of SH‐1028 in EGFR T790M‐positive advanced non–small cell lung cancer patients: A dose‐escalation phase 1 studyPublication Name: CancerPublication Date: 2023-02-22PMID: 36813747DOI: 10.1002/cncr.34697
- SH-1028, An Irreversible Third-Generation EGFR TKI, Overcomes T790M-Mediated Resistance in Non-Small Cell Lung CancerPublication Name: Frontiers in PharmacologyPublication Date: 2021-04-27PMCID: PMC8111447PMID: 33986687DOI: 10.3389/fphar.2021.665253
- [1]. Luwei Han, et al. SH-1028, An Irreversible Third-Generation EGFR TKI, Overcomes T790M-Mediated Resistance in Non-Small Cell Lung Cancer. Front Pharmacol. 2021 Apr 27;12:665253. [Content Brief]
/////////Oritinib, CHINA 2024, APPROVALS 2024, 2035089-28-0, SH 1028, SK593H37SC, rilertinib, Oritinib mesylate, Nanjing Sanhome Pharmaceutical,














