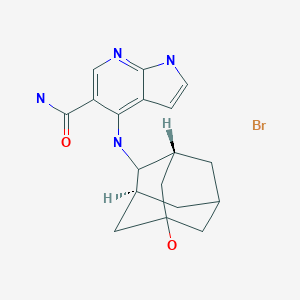
Peficitinib hydrobromide
ペフィシチニブ臭化水素酸塩
ASP015K,
Rheumatoid Arthritis
1H-Pyrrolo(2,3-b)pyridine-5-carboxamide, 4-((5-hydroxytricyclo(3.3.1.13,7)dec-2-yl)amino)-, hydrobromide (1:1), stereoisomer
4-{[(1R,2s,3S,5r)-5
1H-Pyrrolo[2,3-b]py
U55XHZ5X6P
| Formula |
C18H22N4O2. HBr
|
|---|---|
| CAS |
1353219-05-2 HBR
944118-01-8 BASE
|
| Mol weight |
407.3048
|
PMDA, 2019/3/26 JAPAN APPROVED, Smyraf

Peficitinib hydrobromide is used in the treatment of Psoriasis and Rheumatoid Arthritis
Peficitinib (formerly known as ASP015K) is a pyrrolo[2,3-b]pyridine derivative orally administered once-daily JAK inhibitor in development for the treatment of Rheumatoid Arthritis. In preclinical studied Peficitinib inhibited JAK1 and JAK3 with IC50 of 3.9 and 0.7 nM, respectively. Peficitinib also inhibited IL-2-dependent T cell proliferation in vitro and STAT5 phosphorylation in vitro and ex vivo. Furthermore, Peficitinib dose-dependently suppressed bone destruction and paw swelling in an adjuvant-induced arthritis model in rats via prophylactic or therapeutic oral dosing regimens.In clinical trials, Peficitinib treatment prescribed at 50, 100 and 150 mg amounts each showed statistically significantly higher ACR20 response rates compared to the placebo and response rates increased up to the 150 mg dosage. Adverse events included neutropenia, headache, and abdominal pain. The treatment-emergent adverse events occurring more frequently in the Peficitinib group compared with the placebo group included diarrhea, nasopharyngitis, and increased serum creatine phosphokinase activity. No cases of serious infections were reported. Herpes zoster occurred in four patients (two each in the peficitinib 25 and 100 mg cohorts). The authors concluded that treatment with peficitinib as monotherapy for 12 weeks in Japanese patients with moderate to severe RA is efficacious and showed an acceptable safety profile.
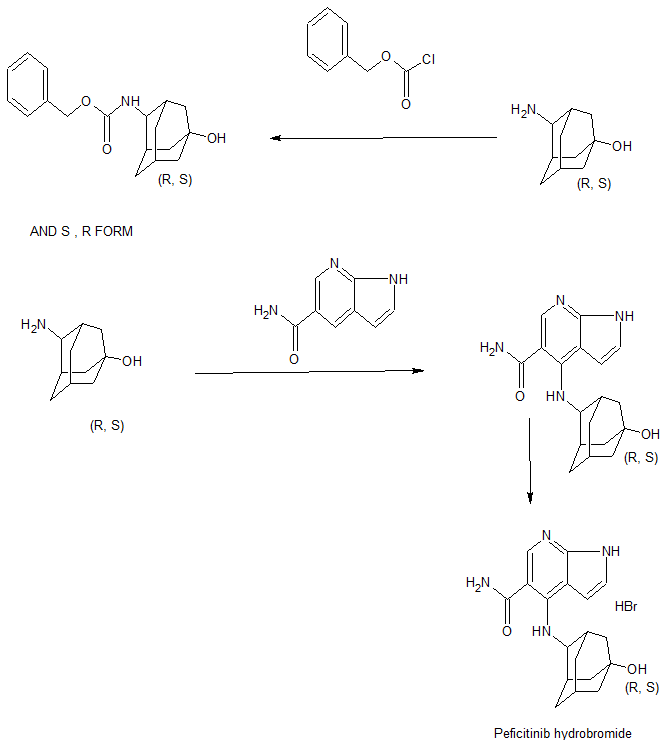
SYN
CLIP
Bioorganic & Medicinal Chemistry
Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor
Abstract
Janus kinases (JAKs) are considered promising targets for the treatment of autoimmune diseases including rheumatoid arthritis (RA) due to their important role in multiple cytokine receptor signaling pathways. Recently, several JAK inhibitors have been developed for the treatment of RA. Here, we describe the identification of the novel orally bioavailable JAK inhibitor 18, peficitinib (also known as ASP015K), which showed moderate selectivity for JAK3 over JAK1, JAK2, and TYK2 in enzyme assays. Chemical modification at the C4-position of lead compound 5 led to a large increase in JAK inhibitory activity and metabolic stability in liver microsomes. Furthermore, we determined the crystal structures of JAK1, JAK2, JAK3, and TYK2 in a complex with peficitinib, and revealed that the 1H-pyrrolo[2,3–b]pyridine-5-carboxamide scaffold of peficitinib forms triple hydrogen bonds with the hinge region. Interestingly, the binding modes of peficitinib in the ATP-binding pockets differed among JAK1, JAK2, JAK3, and TYK2. WaterMap analysis of the crystal structures suggests that unfavorable water molecules are the likely reason for the difference in orientation of the 1H-pyrrolo[2,3-b]pyridine-5-carboxamide scaffold to the hinge region among JAKs.




PATENT
WO 2011162300
[Chemical formula 1]
(Production Method of Hydrobromide Salt Form B 45)
(In the Case of Addition of Seed Crystals )
After nitrogen substitution, the reaction vessel was charged with 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy- 2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide (145.0 kg), water (377 L), EtOH (1508 L), 48% hydrobromic acid (74.9 kg) It charged sequentially at room temperature and started stirring. 48% hydrobromic acid was added, taking care that the pH was in the range of 1.5 to 1.9. The reaction mixture was heated and stirred until the internal temperature reached 70 ° C. or higher. After confirming that the solution was completely dissolved, the solution was stirred for 5 minutes or more, and the solution was subjected to clear filtration at an internal temperature of 70 ° C. or higher, and the pot and line were washed with warm EtOH (290 L). At an internal temperature of about 50 ° C., 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide odor Hydrochloric acid salt seed crystals (B45, 145 g) were added, and the mixture was ripened and stirred overnight at an internal temperature of 40 to 50 ° C. Subsequently, the mixture was cooled to an internal temperature of 20 to 30 ° C. over 1 hour or more, and the mixture was aged and stirred at the same temperature for 1 hour or more. At an internal temperature of 20 to 30 ° C., EtOAc (4350 L) was added dropwise over 1 hour, and the mixture was aged and stirred overnight at the same temperature. The precipitated crystals were filtered. The wet crystals were washed with a solution of EtOH / EtOAc (145 L / 290 L). The wet crystals are dried under reduced pressure at an external temperature of 40 ° C. overnight under reduced pressure to give 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3 -b] Pyridine-5-carboxamide hydrobromide crystal (B45, 161 kg) was obtained.
(In the case of no addition of seed crystals) After
sufficiently drying the reaction vessel and replacing with nitrogen, water (585 L) is charged and subsequently 4- {[ (1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide (225 kg), EtOH (2250 L) was charged Stirring was started. The internal temperature was adjusted to 25 ° C., and 48% hydrobromic acid (127.8 kg) was charged at the same temperature, and the vessel and kettle wall were washed with EtOH (90 L). After the completion of the charging, it was confirmed that the reaction solution had been dissolved, the pH was measured, and the pH was confirmed to be in the range of 1.5 to 1.9. When the pH was out of the range, the pH was adjusted to a predetermined pH using 48% hydrobromic acid (48% hydrobromic acid: about 11.6 kg). The temperature was raised until the internal temperature reached 70 ° C., and after confirmation of dissolution, the mixture was stirred for 5 minutes or more. The solution was subjected to clear filtration while maintaining the internal temperature at 60 ° C. or higher, and washed through a filter from a dissolution vessel with warm EtOH (450 L) preheated to 50 ° C. or higher. The clarified filtrate was gradually cooled to an internal temperature of 45 ° C., and filtered EtOAc (6750 L) was added dropwise over 6 hours at an internal temperature of 45 ° C. After the dropping was completed, the mixture was stirred at an internal temperature of 45 ° C. for 10 hours or more. Subsequently, it was cooled to an internal temperature of 25 ° C. using a follow-up temperature control cooler, and stirred at an internal temperature of 25 ° C. for 3 hours. The predetermined supernatant concentration and the crystal form of the precipitated crystals were confirmed and filtered. A mixed solvent of EtOH / EtOAc (225 L / 450 L) was prepared and cake washed using this mixed solvent. The obtained wet crystals are dried under reduced pressure at an external temperature of 40 ° C. for 10 hours or more, and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [ 2,3-b] pyridine-5-carboxamido hydrobromide crystal (B45, 250 kg) was obtained.
Elemental analysis: theoretical value: C 53.08%, H 5.69% , N 13.76%, O 7.86% , Br 19.62%;
Found:. C 53.02%, H 5.74 %, N 13.73%, Br 19.42%
molecular composition: C 18 H 22 N 4 O 2 . HBr
MS: 327.0 (M From the result of + H) +
elemental analysis, 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5 The carboxamido hydrobromide was a monohydrobromide.
(hydrobromide A87 crystal)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] Pyridine-5-carboxamide (6.0 g) was charged in EtOH / water (57.6 mL / 14.4 mL). At 50-60 ° C., 48% hydrobromic acid was added, stirred for 15 minutes more, and washed with EtOH (18 mL). At 45 ° C.-55 ° C. EtOAc (180 mL) was added dropwise over 30 minutes. Crystals were precipitated upon stirring at 15 ° C to 25 ° C. The crystals were collected by filtration and washed with a mixed solvent of EtOH / EtOAc (6 mL / 12 mL). The crystals are dried under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide odor Seed crystals of hydrofluoride (Form A87, 6.11 g) were obtained.
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide (3.0 g), EtOH (24 mL), water (6 mL), and 48% hydrobromic acid (1.55 g) were charged sequentially at room temperature. After charging, the mixture was heated to an internal temperature of 60 ° C. or higher and stirred. After confirming that the solution was completely in solution, the solution was subjected to clear filtration at an internal temperature of 60 ° C. or higher, and washed with warm EtOH (9 mL). EtOH (21 mL) is added dropwise at an internal temperature of 70 ° C. or higher, and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H at an internal temperature of 70 ° C. Seed crystals (A87, 30 mg) of pyrrolo [2,3-b] pyridine-5-carboxamide hydrobromide were added, and the mixture was ripened and stirred overnight at an internal temperature of 65 to 70 ° C. Subsequently, it was cooled to an internal temperature of 20 to 30 ° C., and ripening stirring was carried out at the same temperature overnight. At an internal temperature of 20 to 30 ° C., EtOAc (90 mL) was added dropwise over 1 hour, and the mixture was aged and stirred at the same temperature for 1 hour or more. The precipitated crystals were collected by filtration. The wet crystals were washed with a solution of EtOH / EtOAc (3 mL / 12 mL). The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide crystal (Form A87, 3.09 g) was obtained.
(hydrobromide A61 crystal)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] Pyridine-5-carboxamide (5.0 g), EtOH (48 mL), water (12 mL), and 48% hydrobromic acid (2.58 g) were charged sequentially at room temperature. After charging, the mixture was heated to an internal temperature of 70 ° C. and stirred. After confirming complete dissolution, the solution was clarified by filtration at an internal temperature of 70 ° C., and washed with warm EtOH (15 mL). The internal temperature was cooled to 50 to 60 ° C., and EtOAc (150 mL) was added dropwise over 1 hour at the same temperature. After the addition was completed, the solution was gradually cooled to 20 to 30 ° C., and the mixture was aged and stirred at the same temperature for 1 hour or more. The precipitated crystals were collected by filtration. The wet crystals were washed with a solution of EtOH / EtOAc (5 mL / 10 mL). The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide crystal (Form A61, 5.19 g) was obtained.
(hydrobromide A36 type crystal)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] To a suspension of pyridine-5-carboxamide (500 mg) in EtOAc, 48% hydrobromic acid (258 μL) was added, and the mixture was stirred with heating under reflux for 1 hour, and further allowed to cool to room temperature. The precipitated crystals were collected by filtration and washed with EtOAc. The resulting crystals are dried at 60 ° C. under reduced pressure to give 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-]. b] Pyridine-5-carboxamide monohydrobromide crystal (Form A36, 625 mg) was obtained.
(B11-type crystal of hydrobromide monohydrate)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2 , 3-b] Pyridine-5-carboxamide (5.0 g), EtOH (48 mL), water (12 mL), 48% hydrobromic acid (2.58 g) were sequentially charged at room temperature. After charging, the mixture was heated to an internal temperature of 70 ° C. or higher and stirred. After confirming that the solution had completely dissolved, the solution was subjected to clear filtration at an internal temperature of 70 ° C. or higher, and washed with warm EtOH (15 mL). 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide odor at an internal temperature of about 35 ° C. Hydrochloric acid salt seed crystals (A87, 49.0 mg) were added, and the mixture was aged with an internal temperature of 30 to 40 ° C. for 4 hours. Subsequently, the mixture was cooled to an internal temperature of 20 to 30 ° C., and aged and stirred overnight at the same temperature. At an internal temperature of 20-25 ° C., EtOAc (150 mL) was added dropwise over 1 hour, and the mixture was aged and stirred at the same temperature for 30 minutes or longer. The precipitated crystals were collected by filtration. The wet crystals were washed with a solution of EtOH / EtOAc (5 mL / 10 mL). The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide monohydrate crystal (Form B11, 5.24 g) was obtained.
(B21-type crystal of hydrobromide dihydrate)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2 , 3-b] Pyridine-5-carboxamide (5.0 g), EtOH (18 mL), water (12 mL), 48% hydrobromic acid (2.58 g) were sequentially charged at room temperature. After charging, the mixture was heated to an internal temperature of 60 ° C. or higher and stirred. After confirming that the solution was completely dissolved, the solution was subjected to clear filtration at an internal temperature of 60 ° C. or higher, and washed with warm EtOH (10 mL). The mixture was cooled to an internal temperature of about 45 to 50 ° C. and aged for 2 hours while stirring. Subsequently, the reaction solution is cooled to an internal temperature of 20 to 30 ° C., and aged at the same temperature and stirred overnight. At an internal temperature of 20 to 30 ° C., EtOAc (160 mL) was added dropwise over 1 hour, and the mixture was aged and stirred for 1 hour or more at the same temperature. The precipitated crystals were filtered. The wet crystals were washed with a solution of EtOH / EtOAc (3 mL / 12 mL). The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide dihydrate crystals (Form B21, 6.05 g) were obtained.
(Tautomerism of each crystal)
(Crystal form conversion; hydrobromide B21 → A61)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H- Pyrrolo [2,3-b] pyridine-5-carboxamide hydrobromide dihydrate (Form B21, 300 mg) and EtOH (3 mL) were sequentially charged at room temperature and suspended overnight. After suspension, the crystals were collected by filtration at room temperature and the wet crystals were washed with EtOH. The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide crystal (Form A61, 258 mg) was obtained.
(Crystal form conversion; hydrobromide B11 → B21)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H- Pyrrolo [2,3-b] pyridine-5-carboxamide hydrobromide monohydrate (form B11, 2.0 g), EtOH (7 mL), water (3 mL) were sequentially charged at room temperature and suspended overnight. It became cloudy. After suspension, the crystals were collected by filtration at room temperature and the wet crystals were washed with 70% aqueous EtOH. The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide dihydrate crystals (Form B21, 1.54 g) were obtained.
(Crystal form conversion; hydrobromide A61 form → B21 form)
4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H- Pyrrolo [2,3-b] pyridine-5-carboxamide hydrobromide (form A61, 1.0 g), EtOH (3.5 mL) and water (1.5 mL) were sequentially charged at room temperature and suspended overnight. After suspension, the crystals were filtered at room temperature and the wet crystals were washed with 70% aqueous EtOH. The wet crystals are dried overnight under vacuum and 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2,3-b] pyridine-5-carboxamide Hydrobromide dihydrate crystals (Form B21, 827 mg) were obtained.
( Example of Preparation of Monohydrate Crystalline Compound (I) Free Form)
4-Chloro-1H-pyrrolo [2,3-b] pyridine-5-carboxamide (44.5 g) under nitrogen atmosphere 1s, 3R, 4s, 5S) -4-aminoadamantan-1-ol (57.0 g) and tributylamine (162.6 mL) were charged in NMP (222.5 mL), and heated and stirred at a bath temperature of 200 ° C. for 2.5 hours. The reaction solution was allowed to cool, and then the reaction solution was added dropwise while stirring in water / Et 2 O (6 L / 0.5 L), followed by stirring for 30 minutes. The obtained solid was collected by filtration, washed twice with water (400 mL), washed twice with Et 2 O (300 mL), and dried. The resulting solid was warmed to dissolve in MeOH (1.8 L) and filtered hot. The resulting mother liquor was concentrated under reduced pressure and MeOH (1.8 L) was added to the residue and heated to dissolve. The resulting solution was allowed to cool and stir, and then stirred at room temperature and aged overnight. The precipitated solid was collected by filtration, washed with EtOH and dried under reduced pressure. The resulting solid was suspended in EtOH (250 mL) and stirred at room temperature for 1 h. The solid was collected by filtration, washed with EtOH and dried under reduced pressure. The obtained solid was suspended in water (900 mL) and stirred at a bath temperature of 70 ° C. for 2 hours. The solid was collected by filtration, washed with water and dried under reduced pressure. Furthermore, the solid was suspended in water (900 mL) and stirred at a bath temperature of 70 ° C. for 2 hours. The solid is collected by filtration, washed with water and then dried under reduced pressure to give 4-{[(1R, 2s, 3S, 5s, 7s) -5-hydroxy-2-adamantyl] amino} -1H-pyrrolo [2 , 3-b] Pyridine-5-carboxamide monohydrate crystal (Form A01, 44 g) was obtained.
REFERENCES
1: D’Amico F, Fiorino G, Furfaro F, Allocca M, Danese S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs. 2018 Jul;27(7):595-599. doi: 10.1080/13543784.2018.1492547. Epub 2018 Jul 6. Review. PubMed PMID: 29938545.
2: Sands BE, Sandborn WJ, Feagan BG, Lichtenstein GR, Zhang H, Strauss R, Szapary P, Johanns J, Panes J, Vermeire S, O’Brien CD, Yang Z, Bertelsen K, Marano C; Peficitinib-UC Study Group. Peficitinib, an Oral Janus Kinase Inhibitor, in Moderate-to-Severe Ulcerative Colitis: Results From a Randomized, Phase 2 Study. J Crohns Colitis. 2018 Jun 15. doi: 10.1093/ecco-jcc/jjy085. [Epub ahead of print] PubMed PMID: 29917064.
3: Veale DJ, McGonagle D, McInnes IB, Krueger JG, Ritchlin CT, Elewaut D, Kanik KS, Hendrikx T, Berstein G, Hodge J, Telliez JB. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology (Oxford). 2018 Apr 3. doi: 10.1093/rheumatology/key070. [Epub ahead of print] PubMed PMID: 29618084.
4: Cline A, Cardwell LA, Feldman SR. Advances in treating psoriasis in the elderly with small molecule inhibitors. Expert Opin Pharmacother. 2017 Dec;18(18):1965-1973. doi: 10.1080/14656566.2017.1409205. Epub 2017 Nov 27. Review. PubMed PMID: 29171774.
5: Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018 Feb;77(2):175-187. doi: 10.1136/annrheumdis-2017-211555. Epub 2017 Aug 1. Review. PubMed PMID: 28765121.
6: Zhu T, Howieson C, Wojtkowski T, Garg JP, Han D, Fisniku O, Keirns J. The Effect of Verapamil, a P-Glycoprotein Inhibitor, on the Pharmacokinetics of Peficitinib, an Orally Administered, Once-Daily JAK Inhibitor. Clin Pharmacol Drug Dev. 2017 Nov;6(6):548-555. doi: 10.1002/cpdd.344. Epub 2017 Mar 16. PubMed PMID: 28301084.
7: Genovese MC, Greenwald M, Codding C, Zubrzycka-Sienkiewicz A, Kivitz AJ, Wang A, Shay K, Wang X, Garg JP, Cardiel MH. Peficitinib, a JAK Inhibitor, in Combination With Limited Conventional Synthetic Disease-Modifying Antirheumatic Drugs in the Treatment of Moderate-to-Severe Rheumatoid Arthritis. Arthritis Rheumatol. 2017 May;69(5):932-942. doi: 10.1002/art.40054. PubMed PMID: 28118538.
8: Ito M, Yamazaki S, Yamagami K, Kuno M, Morita Y, Okuma K, Nakamura K, Chida N, Inami M, Inoue T, Shirakami S, Higashi Y. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017 Jan;133(1):25-33. doi: 10.1016/j.jphs.2016.12.001. Epub 2016 Dec 23. PubMed PMID: 28117214.
9: Zhu T, Parker B, Wojtkowski T, Nishimura T, Garg JP, Han D, Fisniku O, Keirns J. Drug Interactions Between Peficitinib, an Orally Administered, Once-Daily Janus Kinase Inhibitor, and Rosuvastatin in Healthy Subjects. Clin Pharmacokinet. 2017 Jul;56(7):747-757. doi: 10.1007/s40262-016-0474-4. PubMed PMID: 27878567.
10: Semerano L, Decker P, Clavel G, Boissier MC. Developments with investigational Janus kinase inhibitors for rheumatoid arthritis. Expert Opin Investig Drugs. 2016 Dec;25(12):1355-1359. Epub 2016 Oct 31. PubMed PMID: 27748152.
11: Kivitz AJ, Gutierrez-Ureña SR, Poiley J, Genovese MC, Kristy R, Shay K, Wang X, Garg JP, Zubrzycka-Sienkiewicz A. Peficitinib, a JAK Inhibitor, in the Treatment of Moderate-to-Severe Rheumatoid Arthritis in Patients With an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2017 Apr;69(4):709-719. doi: 10.1002/art.39955. PubMed PMID: 27748083.
12: Lam S. JAK inhibitors: A broadening approach in rheumatoid arthritis. Drugs Today (Barc). 2016 Aug;52(8):467-469. PubMed PMID: 27722215.
13: Roskoski R Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016 Sep;111:784-803. doi: 10.1016/j.phrs.2016.07.038. Epub 2016 Jul 26. Review. PubMed PMID: 27473820.
14: Iwata S, Tanaka Y. Progress in understanding the safety and efficacy of Janus kinase inhibitors for treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2016 Oct;12(10):1047-57. doi: 10.1080/1744666X.2016.1189826. Epub 2016 Jun 6. Review. PubMed PMID: 27253519.
15: Cao YJ, Sawamoto T, Valluri U, Cho K, Lewand M, Swan S, Lasseter K, Matson M, Holman J Jr, Keirns J, Zhu T. Pharmacokinetics, Pharmacodynamics, and Safety of ASP015K (Peficitinib), a New Janus Kinase Inhibitor, in Healthy Subjects. Clin Pharmacol Drug Dev. 2016 Nov;5(6):435-449. doi: 10.1002/cpdd.273. Epub 2016 Jun 30. PubMed PMID: 27162173.
16: Nielsen OH, Seidelin JB, Ainsworth M, Coskun M. Will novel oral formulations change the management of inflammatory bowel disease? Expert Opin Investig Drugs. 2016 Jun;25(6):709-18. doi: 10.1517/13543784.2016.1165204. Epub 2016 Mar 28. Review. PubMed PMID: 26967267.
17: Yiu ZZ, Warren RB. Novel Oral Therapies for Psoriasis and Psoriatic Arthritis. Am J Clin Dermatol. 2016 Jun;17(3):191-200. doi: 10.1007/s40257-016-0179-3. Review. PubMed PMID: 26923915.
18: Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016 Jun;75(6):1057-64. doi: 10.1136/annrheumdis-2015-208279. Epub 2015 Dec 15. PubMed PMID: 26672064; PubMed Central PMCID: PMC4893099.
19: Oda K, Cao YJ, Sawamoto T, Nakada N, Fisniku O, Nagasaka Y, Sohda KY. Human mass balance, metabolite profile and identification of metabolic enzymes of [¹⁴C]ASP015K, a novel oral janus kinase inhibitor. Xenobiotica. 2015;45(10):887-902. doi: 10.3109/00498254.2015.1026864. Epub 2015 May 19. PubMed PMID: 25986538.
20: Nakada N, Oda K. Identification and characterization of metabolites of ASP015K, a novel oral Janus kinase inhibitor, in rats, chimeric mice with humanized liver, and humans. Xenobiotica. 2015;45(9):757-65. doi: 10.3109/00498254.2015.1019594. Epub 2015 Jun 12. PubMed PMID: 25869242.
/////////////////Peficitinib hydrobromide, Smyraf, JAPAN 2019, ペフィシチニブ臭化水素酸塩 , ASP015K, Rheumatoid Arthritis
O=C(C1=CN=C(NC=C2)C2=C1N[C@@H]3[C@]4([H])C[C@@]5([H])C[C@](C4)(O)C[C@]3([H])C5)N.[H]Br