PF 4136309
PF4136309; PF 4136309; PF-4136309; PF04136309; PF4136309; PF-04136309; INCB8761; INCB 8761; INCB-8761
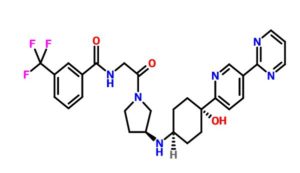
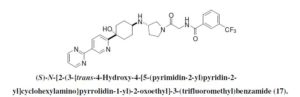
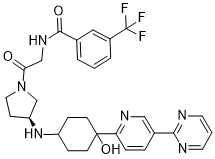
(S)-N-(2-(3-((4-hydroxy-4-(5-(pyrimidin-2-yl)pyridin-2-yl)cyclohexyl)amino)pyrrolidin-1-yl)-2-oxoethyl)-3-(trifluoromethyl)benzamide
N-[2-[(3S)-3-[[trans-4-Hydroxy-4-[5-(2-pyrimidinyl)-2-pyridinyl]cyclohexyl]amino]-1-pyrrolidinyl]-2-oxoethyl]-3-(trifluoromethyl)benzamide
N-[2-((3S)-3-[4-hydroxy-4-(4-pyrimidin-2-ylphenyl)cyclohexyl]aminopyrrolidin-1-yl)-2- oxoethyl]-3-(trifluoromethyl)benzamide
1341224-83-6
MF: C29H31F3N6O3
MW: 568.24097
CC chemokine receptor 2 (CCR2) antagonist


PF-4136309, also known as INCB8761, is an orally available human chemokine receptor 2 (CCR2) antagonist with potential immunomodulating and antineoplastic activities. Upon oral administration, CCR2 antagonist PF-04136309 specifically binds to CCR2 and prevents binding of the endothelium-derived chemokine ligand CLL2 (monocyte chemoattractant protein-1 or MCP1) to its receptor CCR2, which may result in inhibition of CCR2 activation and signal transduction. This may inhibit inflammatory processes as well as angiogenesis, tumor cell migration, and tumor cell proliferation. The G-protein coupled receptor CCR2 is expressed on the surface of monocytes and macrophages, stimulates the migration and infiltration of these cell types, and plays an important role in inflammation, angiogenesis, and tumor cell migration and proliferation.
- Originator Pfizer
- Class Analgesics
- Mechanism of Action CCR2 receptor antagonists
Highest Development Phases
- Phase I/II Pancreatic cancer
- Discontinued Hepatic fibrosis; Pain
Most Recent Events
- 01 Apr 2016 Phase-I/II clinical trials in Pancreatic cancer (Combination therapy, First-line therapy, Metastatic disease) in USA (PO) (NCT02732938)
- 01 Dec 2015 Phase-I clinical trials in Pancreatic cancer (In volunteers) in Belgium (PO) (NCT02598206)
- 09 Nov 2015 Pfizer plans a phase I trial in Healthy volunteers in Belgium and USA (NCT02598206)
(S)-N-[2-(3-{trans-4-Hydroxy-4-[5-(pyrimidin-2-yl)pyridin-2-
yl]cyclohexylamino}pyrrolidin-1-yl)-2-oxoethyl]-3-(trifluoromethyl)benzamide
MS (M+H)+:569.2.
1H NMR (400 MHz, CD3OD): δ 9.57 – 9.45 (m, 1H), 8.94-8.84 (m, 2H), 8.82 –
8.72 (m, 1H), 8.27 – 8.19 (m, 1H), 8.15 (d, J = 7.8 Hz, 1H), 7.91 – 7.84 (m, 2H), 7.69
(dd, J = 7.8, 7.8 Hz, 1H), 7.46-7.39 (m, 1H), 4.29 – 4.12 (m, 2H), 3.87 (dd, J = 10.1, 6.4
Hz, 0.5H), 3.83 – 3.39 (m, 3.5H), 3.38 – 3.32 (m, 1H), 3.02 – 2.91 (m, 1H), 2.51 – 2.35
(m, 2H), 2.34 – 2.14 (m, 1H), 2.13 – 1.88 (m, 2.5H), 1.88 – 1.76 (m, 0.5H), 1.74 – 1.56
(m, 4H).
Anal. (C29H31F3N6O3): calcd C 61.24, H 5.50, N 14.79; found C 61.18, H 5.59,
N 14.87.
INTERMEDIATES
8-(5-Bromopyridin-2-yl)-1,4-dioxaspiro[4.5]decan-8-ol
LC-MS (M+H)+: 316.1/314.1. 1H NMR (300 MHz,CDCl3): δ 8.60 (s, 1 H), 7.82 (d, 1 H), 7.38 (d, 1 H), 4.6 (s, 1 H), 4.0 (m, 4 H), 2.2 (m, 4
H), 1.7 (m, 4 H).
8-(5-Pyrimidin-2-ylpyridin-2-yl)-1,4-dioxaspiro[4.5]decan-8-ol
LC-MS (M+H)+: 314.2.
4-Hydroxy-4-(5-pyrimidin-2-ylpyridin-2-yl)cyclohexanone
MS
(M+H)+: 270.2.
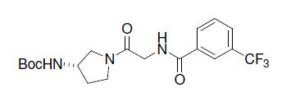
tert-Butyl [(S)-1-({[3-(Trifluoromethyl)benzoyl]amino}acetyl)
pyrrolidin-3-yl]carbamate.
MS (M-Boc+H)+: 316.
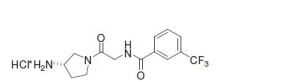
(S)-N-{2-[3-Aminopyrrolidin-1-yl]-2-oxoethyl}-3-(trifluoromethyl)
benzamide hydrochloride
MS
(M+H)+: 316.
PATENT
WO 2012114223
https://www.google.com/patents/WO2012114223A1?cl=en
Example 35
Step A
8-(4-lodo-phenyl)-1 ,4-dioxa-spiro[4.5]decan-8-ol. To a solution of 1 ,4-diiodobenzene (16.5 g, 50 mmol) in THF (350 mL) at -78°C was added n-BuLi (2.5 M, 24 mL) over 1 hour. After stirred additional 30 minutes, a solution of 1 ,4-dioxa-spiro[4.5]decan-8-one (7.8 g, 50 mmol) in THF (30 mL) was added in and the resulting mixture was stirred for 3 hours. To the mixture was added TMSCI (5.4 g, 50 mmol) and the resulting mixture was allowed to warm to rt and stirred at rt for 18 hours. The reaction mixture was neutralized to pH 6.0, and extracted with ethyl acetate (3X 50 mL). The organic extracts were combined, washed with saline solution (2X 50 mL), dried over sodium sulfate, concentrated in vacuo. The residue was chromatographed on silica gel, eluting with hexane/ethyl acetate (95/5 to 100/0). The appropriate fractions were combined to give 8-(4-lodo-phenyl)-1 ,4-dioxa-spiro[4.5]decan-8-ol (12 g, 66.6%) with LCMS: 361 .2 (M+H+, 100%) and {[8-(4-iodophenyl)-1 ,4- dioxaspiro[4.5]dec-8-yl]oxy}(trimethyl)silane (6 g, 27%) with LCMS: 433.1 (M+H+, 100%). Step B
8-(4-pyrimidin-2-ylphenyl)-1 ,4-dioxaspiro[4.5]decan-8-ol. To a solution of 8-(4-iodo- phenyl)-1 ,4-dioxa-spiro[4.5]decan-8-ol (450.0 mg, 1.249 mmol) in THF (1.0 mL) at room temperature was added dropwise isopropylmagnesium chloride (2.0 M in THF, 1 .37 mL) and the reaction mixture was stirred at room temperature for 30 mins. To another flask charged with nickel acetylacetonate (20 mg, 0.06 mmol) and 1 ,3-bis(diphenylphosphino)-propane (26 mg, 0.062 mmol) suspened in THF (3 mL) under N2 was added 2-bromopyrimidine (199 mg, 1.25 mmol). The resulting mixture was stirred at room temperature until it is clear. The second mixture was transferred into the degassed Grignard solution prepared in step 1. The resulting mixture was stirred at room temperature overnight. The reaction mixture was diluted with EtOAc, quenched with water, washed with brine, dried overNa2S04, and concentrated. The residue was columned on silica gel, eluted with hexane/EtOAc (2/1 ), to gave the desired compound (270 mg, 69%) as white solid. LCMS: 313.1 , (M+H, 100%). 1H
NMR (CDCIs): δ 8.86 (d, 2H), 8.46 (dd, 2H), 7.71 (dd, 2H), 7.24 (t, 1 H), 4.05 (d, 4H), 2.30 (dt, 2H), 2.18 (dt, 2H), 1 .90 (m, 2H), 1 .78 (m, 2H).
Step C
4-Hydroxy-4-(4-pyrimidin-2-ylphenyl)cyclohexanone. The title compound was prepared by treating the ketal of step B with HCI in water following the procedure described in step B of Example 2. MS (M+H)+ 269.
Step D
N-[2-((3S)-3-[4-hydroxy-4-(4-pyrimidin-2-ylphenyl)cyclohexyl]aminopyrrolidin-1-yl)-2- oxoethyl]-3-(trifluoromethyl)benzamide bis(trifluoroacetate) (salt). To a 1-neck round-bottom flask charged with methylene chloride (1 ml.) was added 4-hydroxy-4-(4-pyrimidin-2- ylphenyl)cyclohexanone (50.0 mg, 0.186 mmol), N-2-[(3S)-3-aminopyrrolidin-1-yl]-2- oxoethyl-3-(trifluoromethyl)benzamide hydrochloride (65.5 mg, 0.186 mmol), and triethylamine (85.7 uL, 0.615 mmol). The resulting mixture was stirred at 25°C for 30 minutes, and to it was added sodium triacetoxyborohydride (62.4 mg, 0.28 mmol) in portion. The reaction mixture was stirring at rt overnight. The reaction was concentrated, and the residue was chromatographed on Si02, eluted with acetone/methanol (100% to 90%/10%) to give two fractions, which were further purified on prep-LCMS separately to afford F1 (24.2 mg ) and F2 (25.9 mg) as white powder in total 34% of the yield. LCMS: 568.2 (M+H, 100%)
Paper
Discovery of INCB8761/PF-4136309, a Potent, Selective, and Orally Bioavailable CCR2 Antagonist

We report the discovery of a new (S)-3-aminopyrrolidine series of CCR2 antagonists. Structure–activity relationship studies on this new series led to the identification of 17 (INCB8761/PF-4136309) that exhibited potent CCR2 antagonistic activity, high selectivity, weak hERG activity, and an excellent in vitro and in vivo ADMET profile. INCB8761/PF-4136309 has entered human clinical trials.
HPLC
http://link.springer.com/article/10.1007/s10337-015-2860-8
A precise and sensitive LC method was developed and further validated for the determination of enantiomeric purity of (S)-N-[2-(3-{trans-4-hydroxy-4-[5-(pyrimidin-2-yl)pyridin-2-yl] cyclohexylamino} pyrrolidin-1-yl)-2-oxoethyl]-3-(trifluoromethyl) benzamide (PF-04136309). Baseline separation with a resolution higher than 1.8 was accomplished within 40 min using a CHIRALPAK AD (250 × 4.6 mm; particle size 5 μm) column, with n-hexane:2-propanol (70:30v/v) as mobile phase at a flow rate of 1 mL min−1. The eluted analytes were subsequently detected with a UV detector at 260 nm. The effects of mobile phase components and temperature on enantiomeric selectivity as well as the resolution of enantiomers were thoroughly investigated. The calibration curves were plotted within a concentration range between 0.01 and 1 mg mL−1 (n = 9), and recoveries between 98.17 and 101.28 % were obtained, with relative standard deviation (RSD) lower than 1.44 %. The LOD and LOQ for PF-04136309 were 3.59 and 11.54 μg mL−1 and for its enantiomer were 3.39 and 11.28 μg mL−1, respectively. The developed method was demonstrated to be accurate, robust and sensitive for the determination of enantiomeric purity of PF-04136309, especially for the analysis of bulk samples.
REFERENCES
1: Xue CB, Wang A, Han Q, Zhang Y, Cao G, Feng H, Huang T, Zheng C, Xia M, Zhang K, Kong L, Glenn J, Anand R, Meloni D, Robinson DJ, Shao L, Storace L, Li M, Hughes RO, Devraj R, Morton PA, Rogier DJ, Covington M, Scherle P, Diamond S, Emm T, Yeleswaram S, Contel N, Vaddi K, Newton R, Hollis G, Metcalf B. Discovery of INCB8761/PF-4136309, a Potent, Selective, and Orally Bioavailable CCR2 Antagonist. ACS Med Chem Lett. 2011 Oct 5;2(12):913-8. doi: 10.1021/ml200199c. eCollection 2011 Dec 8. PubMed PMID: 24900280; PubMed Central PMCID: PMC4018168.
http://www.pfizer.com/files/news/asco/ASCO2016_PipelineFactSheet_CCR2.pdf
//////1341224-83-6, PF 4136309, PF4136309, PF 4136309, PF-4136309, PF04136309, PF4136309, PF-04136309, INCB8761, INCB 8761, INCB-8761, PFIZER, PHASE 2
O=C(NCC(N1C[C@@H](NC2CCC(C3=NC=C(C4=NC=CC=N4)C=C3)(O)CC2)CC1)=O)C5=CC=CC(C(F)(F)F)=C5